|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Morphine is the oldest, commonly used long-acting narcotic. Its use in neonates remains controversial because early studies in newborn infants suggested that morphine produces greater respiratory depression than meperidine does. Higher brain levels of morphine were found in neonatal than adult rats, which suggests that permeability of the blood-brain barrier may account in part for the apparent sensitivity of the human neonate to morphine.[171] This rationale led to the common belief that infants are sensitive to the effect of narcotics. More recent studies have found age-dependent pharmacokinetics. The newborn has lower clearance of morphine, and therefore a lower dose will result in higher plasma values because of a longer elimination half-life.[172] Term infants older than 10 days may clear morphine more rapidly and at a similar rate as adults.
The issues of respiratory sensitivity and the age at which it decreases in humans have yet to be resolved; animal studies suggest that there appears to be a difference between morphine and fentanyl that may not relate to transport of drug into the brain.[173] Some of these effects may therefore be due to changes in pharmacodynamics rather than simply maturation of the blood-brain barrier. Morphine should be administered with caution in neonates and premature infants who are not in a monitored setting. Infants older than 6 months probably have a normal adult response to morphine.
Meperidine received great attention when it was found on a milligram-per-kilogram basis to cause less respiratory depression in newborns than morphine does.[174] This difference may be related in part to the fact that meperidine is much more lipophilic than morphine. The age-dependent difference in blood-brain barrier permeability would be much less important for meperidine. Unlike morphine, the fraction of drug that enters the brain of neonates is similar to that entering the brain of older children. Like all drugs administered to neonates, there is large patient-to-patient variability in metabolism and response.[175] Meperidine is not appropriate for long-term administration because of accumulation of the toxic metabolite normeperidine.[176]
Fentanyl is the most commonly used narcotic in infants and children. [177] Its major advantages relate to its rapid onset and brief duration of action. This narcotic is more lipophilic than meperidine; the potential effects of the blood-brain barrier are of no importance with fentanyl. Termination of the effect of low doses of fentanyl results primarily from redistribution, whereas termination of the effect of high doses depends on elimination. High doses of fentanyl achieve properties of long-acting narcotics.
Fentanyl induces a very stable cardiovascular response while providing an anesthetic state. The dosage required to produce anesthesia varies considerably, depending on patient age, the surgical procedure, the health of the patient, and the use of anesthetic adjuvants.[178] Neonates undergoing abdominal surgery have a longer fentanyl half-life than do neonates undergoing other procedures; hepatic blood flow—and factors that greatly increase or decrease hepatic blood flow such as positive end-expiratory pressure or the use of vasopressors—may alter the pharmacokinetics of this drug.[179] Impaired hepatic function may also play a role in altered kinetics with increased intra-abdominal pressure.[180] Therefore, the pharmacokinetic and pharmacodynamic profile is very different and more variable for neonates than for older children. A dose of 12.5 µg/kg produces anesthesia in full-term neonates undergoing abdominal surgery, whereas higher doses (30 to 100 µg/kg) are used for cardiac surgery. These doses are safe in children whose ventilation will be controlled post-operatively; much lower doses (2 to 10 µg/kg) should be
Oral transmucosal administration (5 to 15 µg/kg, maximum of 400 µg) results in reasonably rapid absorption, with peak blood levels achieved within 15 to 30 minutes.[181] Children have lower bioavailability than adults do.[182] Because vomiting and desaturation have been reported preoperatively, this form of administration will be of greater use for painful procedures outside the operating room and for postoperative analgesia.[183] [184] [185] [186] [187] Our experience has been that the incidence of serious adverse effects (desaturation, vomiting) before anesthetic induction was minimal if anesthesia is induced in children within 10 minutes of completion of the Fentanyl Oralet.[188] [189] It is likely that the incidence of adverse effects increases with a longer time between completion and induction of anesthesia; these effects may also relate to the dose (µg/kg) administered. A recent study has found that the liquid intravenous formulation is rapidly absorbed. Similar serum concentrations are achieved when similar doses as those recommended for the Fentanyl Oralet are administered ( Fig. 60-7 ). [190] Oral administration of the intravenous formulation may be a reasonable substitute for intramuscular opioid administration in children who do not have intravenous access because the elimination curve is long and relatively flat. Another advantage may be the shorter and less variable consumption time and greater versatility in dosing because the patient dose can be more accurately titrated than the Fentanyl Oralet can.
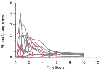 Figure 60-7
Plasma fentanyl levels in 10 children who ingested the
liquid intravenous formulation of fentanyl. The peak plasma levels achieved were
nearly identical to those achieved with a similar dose of the Fentanyl Oralet.[181]
[182]
Like the Fentanyl Oralet, there was large
patient-to-patient variability and a long slow elimination curve. The advantage
is that the intravenous formulation is much cheaper and there is no problem with
variable consumption time. (Redrawn from Wheeler M, Birmingham PK, Lugo
RA, et al: Pharmacokinetics of the intravenous formulation of fentanyl citrate administered
orally in children undergoing general anesthesia. Anesth Analg [in press].)
Figure 60-7
Plasma fentanyl levels in 10 children who ingested the
liquid intravenous formulation of fentanyl. The peak plasma levels achieved were
nearly identical to those achieved with a similar dose of the Fentanyl Oralet.[181]
[182]
Like the Fentanyl Oralet, there was large
patient-to-patient variability and a long slow elimination curve. The advantage
is that the intravenous formulation is much cheaper and there is no problem with
variable consumption time. (Redrawn from Wheeler M, Birmingham PK, Lugo
RA, et al: Pharmacokinetics of the intravenous formulation of fentanyl citrate administered
orally in children undergoing general anesthesia. Anesth Analg [in press].)
Alfentanil is eliminated more rapidly than fentanyl; its pharmacokinetics is independent of dose.[191] This property may provide a margin of safety because the greater the administered dose, the greater the elimination. Clearance of alfentanil may be increased in children in comparison to adults.[192] As with any narcotic, there is important patient-to-patient variability in pharmacokinetics and pharmacodynamics in neonates and in patients with impaired hepatic blood flow.[193] [194]
Sufentanil has been used primarily for cardiac anesthesia; age-dependent kinetics is also evident, particularly during the first month of life.[195] Children are able to clear sufentanil more rapidly than adults do. This drug must be administered with caution because severe bradycardia and asystole have been reported when a vagolytic drug was not administered simultaneously.[196] This narcotic has also been administered nasally as a premedicant (2 µg/kg); however, desaturation may follow.[165]
Remifentanil is the most recent addition to the opioids available for the care of children.[197] The main advantage of this opioid is its very brief half-life. Studies in adults have found that even after prolonged infusion, the time to 50% reduction in effect-site concentration (effect on respiration) is approximately 4 minutes.[198] One study examining its pharmacokinetics in children found age-related differences in volume of distribution and clearance but not half-life ( Fig. 60-8 ).[199] Contrary to the pharmacokinetics of most drugs, neonates are able to clear the drug more rapidly than older patients are! This difference may in part relate to the larger volume of distribution with equivalent half-life. Of further interest is the very small patient-to-patient variability in pharmacokinetic parameters when compared with similar studies examining other opioids, particularly in infants and neonates.[195] [200] [201] Because remifentanil is broken down by nonspecific plasma and tissue cholinesterases, the importance of maturation of renal and hepatic function is minimal. This also helps explain the minimal difference in remifentanil's half-life between infants and adults. This drug would appear to also have great utility in infants with hepatic or renal failure.[202] The particularly favorable pharmacokinetics in neonates may prove to be very useful in infants when a deep plane of anesthesia is needed while avoiding cardiovascular depression and still avoid postoperative ventilation. One multicenter study of infants undergoing pyloromyotomy, however, found no difference in intraoperative hemodynamic parameters, time to extubation, postanesthesia care unit discharge times, need for pain medications, or adverse events when compared with halothane anesthesia.[203] [204] In older children, remifentanil will be helpful in the anesthetic management of those in whom neurologic status must be rapidly assessed.[202] [205] [206] This drug may also be very useful in cardiac surgical patients as a means of providing adequate opioid analgesia, cardiovascular stability, and early extubation.[207]
Bolus doses of remifentanil are associated with hypotension, chest wall rigidity, and bradycardia.[208] Therefore, a
 Figure 60-8
Remifentanil is the newest potent opioid available for
the care of neonates. Note that unlike virtually all other medications, the half-life
is shorter in neonates than in older children, probably because of remifentanil's
elimination by nonspecific plasma and tissue esterases, as well as the larger volume
of distribution in neonates. The importance of this observation is that developmental
immaturity of liver and renal function does not affect remifentanil pharmacokinetics.
Because this was a single-bolus administration study, it is not clear whether similar
pharmacokinetics would follow a long continuous infusion. (Data abstracted
from Ross AK, Davis PJ, et al: Pharmacokinetics of remifentanil in anesthetized
pediatric patients undergoing elective surgery or diagnostic procedures. Anesth
Analg 93:1393–1401, 2001.)
Figure 60-8
Remifentanil is the newest potent opioid available for
the care of neonates. Note that unlike virtually all other medications, the half-life
is shorter in neonates than in older children, probably because of remifentanil's
elimination by nonspecific plasma and tissue esterases, as well as the larger volume
of distribution in neonates. The importance of this observation is that developmental
immaturity of liver and renal function does not affect remifentanil pharmacokinetics.
Because this was a single-bolus administration study, it is not clear whether similar
pharmacokinetics would follow a long continuous infusion. (Data abstracted
from Ross AK, Davis PJ, et al: Pharmacokinetics of remifentanil in anesthetized
pediatric patients undergoing elective surgery or diagnostic procedures. Anesth
Analg 93:1393–1401, 2001.)
For practical purposes and for safety, it is vital that the drug be administered by continuous infusion and that it be carried into the vein by a second continuous infusion device because variations in drug delivery related to variations in intravenous fluid delivery can have profound effects on the rate of opioid administration. I generally use a continuous infusion pump for the maintenance intravenous fluid and "piggyback" the remifentanil infusion as close to the intravenous catheter as possible. In complex cases I will use a separate intravenous line for all other anesthetic management issues. A further very important concern is the need to provide analgesia once the remifentanil is discontinued. I usually accomplish this with morphine (0.05 to 0.2 mg/kg, depending on the severity of the expected pain) or with a regional nerve block administered well before the discontinuation of remifentanil.
|
|
|
|
|
|
|
|
|
|
|
|
|