HEMODYNAMIC PROBLEMS DURING LAPAROSCOPY
Hemodynamic changes observed during laparoscopy result from the
combined effects of pneumoperitoneum, patient position, anesthesia, and hypercapnia
from the absorbed CO2
. In addition to these pathophysiologic changes,
reflex increases of vagal tone and arrhythmias can develop.
Hemodynamic Repercussions of Pneumoperitoneum in Healthy
Patients
Peritoneal insufflation to IAPs more than 10 mm Hg induces significant
alterations of hemodynamics.[71]
[72]
[73]
[74]
[75]
These disturbances are characterized by decreases of cardiac output, elevations
of arterial pressure, and increases of systemic and pulmonary vascular resistances.
Heart rate remains unchanged or increases only slightly. The decrease in cardiac
output is proportional to the increase in IAP.[76]
[77]
Cardiac output has also been reported to be
increased[78]
or unchanged during pneumoperitoneum.
[79]
[80]
[81]
These discrepancies may be caused by differences in rates of CO2
insufflation,
IAP,[82]
steepness of patient tilt, time intervals
between insufflation and collection of data, techniques used to assess hemodynamics,
and anesthetic techniques. However, most studies have shown a fall of cardiac output
(10% to 30%) during peritoneal insufflation whether the patient was placed in the
head-down[83]
[84]
[85]
or head-up position.[86]
[87]
[88]
These
adverse hemodynamic effects of pneumoperitoneum have been confirmed by studies using
pulmonary artery catheterization,[85]
[86]
[88]
thoracic electrical bioimpedance,[84]
[87]
esophageal echo-Doppler,[89]
and transesophageal echocardiography.[90]
[91]
[92]
Normal intraoperative values of venous oxygen
saturation (SVO2
) and lactate concentrations
suggest that changes in cardiac output occurring during pneumoperitoneum are well
tolerated by healthy patients.[79]
[88]
Cardiac output, which decreases shortly after the beginning of peritoneal insufflation,
subsequently increases, probably as a result of surgical stress.[87]
[88]
Hemodynamic degradation occurs mainly at the
beginning of peritoneal insufflation.
The mechanism of the decrease of cardiac output is probably multifactorial
( Fig. 57-5
). A decrease
in venous return is observed after a transient increase in venous return seen at
low IAPs (<10 mm Hg).[76]
[82]
[93]
[94]
Increased
IAP results in caval compression,[95]
pooling of
blood in the legs,[96]
and an increase in venous
resistance.[93]
The decline in venous return, which
parallels the decrease in cardiac output,[76]
[77]
is confirmed by a reduction in left ventricular end-diastolic volume measured using
transesophageal echocardiography.[90]
Cardiac filling
pressures, however, rise during peritoneal insufflation.[76]
[85]
[86]
[88]
The paradoxical increase of these pressures can be explained by the increased intrathoracic
pressure associated with pneumoperitoneum.[76]
[86]
[87]
[97]
Right
atrial pressure and pulmonary artery occlusion pressure can no longer be considered
reliable indices of cardiac filling pressures during pneumoperitoneum. The fact
that atrial natriuretic peptide concentrations remain low despite increased pulmonary
capillary occlusion pressure during pneumoperitoneum further suggests that abdominal
insufflation interferes with venous return.[98]
The reduction in venous return and cardiac output can be attenuated by increasing
circulating volume before the pneumoperitoneum[93]
[99]
( Fig.
57-6
). Increased filling pressures can be achieved by fluid loading or
tilting the patient to a slight head-down position before peritoneal insufflation,
by preventing pooling of blood with intermittent sequential pneumatic compression
device,[100]
or by wrapping the legs with elastic
bandages.[101]
Although inotropism is difficult to assess,[91]
the ejection fraction of the left ventricle assessed by echocardiography does not
appear to decrease significantly when IAP increases to 15 mm Hg.[90]
[102]
However, all studies reported describe an
increase in systemic vascular resistance during pneumoperitoneum. This increase
in afterload cannot be considered simply to be a reflex sympathetic response to
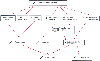 Figure 57-5
Schematic representation of the different mechanisms
leading to decreased cardiac output during pneumoperitoneum for laparoscopy.
Figure 57-5
Schematic representation of the different mechanisms
leading to decreased cardiac output during pneumoperitoneum for laparoscopy.
decreased cardiac output.[86]
[102]
Systemic vascular resistance also increased in studies in which no decrease in cardiac
output was reported.[79]
[102]
Whereas the normal heart tolerates increases in afterload under physiologic conditions,
the changes in afterload produced by pneumoperitoneum can result in deleterious effects
in patients with cardiac diseases and may lead to further decreases in cardiac output.
[103]
The increase in systemic vascular resistance
is
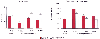 Figure 57-6
Changes in the cardiac index and systemic vascular resistance
during laparoscopy in two groups of patients. For group 1 (controls, n = 10, filled
bars), pneumoperitoneum was induced with patients in a 10-degree head-up
position. Group 2 (volume loaded, n = 10, open bars)
patients received 500 mL of lactated Ringer's solution before anesthesia induction
and were insufflated in the supine position. Data are presented as the mean ±
SEM.
Figure 57-6
Changes in the cardiac index and systemic vascular resistance
during laparoscopy in two groups of patients. For group 1 (controls, n = 10, filled
bars), pneumoperitoneum was induced with patients in a 10-degree head-up
position. Group 2 (volume loaded, n = 10, open bars)
patients received 500 mL of lactated Ringer's solution before anesthesia induction
and were insufflated in the supine position. Data are presented as the mean ±
SEM.
affected by patient position. Whereas the Trendelenburg position attenuates this
increase, the head-up position aggravates it.[79]
[80]
[85]
[98]
The patient's circulating volume affects changes in venous return and changes in
afterload. The increase in systemic vascular resistance can be corrected by administration
of vasodilating anesthetic agents, such as isoflurane,[86]
or direct vasodilating drugs, such as nitroglycerin[104]
or nicardipine.[105]
The increase in systemic vascular resistance is considered to
be mediated by mechanical and neurohumoral factors.[72]
The return of hemodynamic variables to baseline is gradual and takes several minutes,
suggesting the involvement of neurohumoral factors.[84]
[86]
[103]
Catecholamines,
the renin-angiotensin system, and especially vasopressin are all released during
pneumoperitoneum and may contribute to increasing afterload.[87]
[88]
[97]
[98]
[106]
[107]
However,
only the time course of vasopressin release parallels that of systemic vascular resistance.
[87]
[88]
[107]
Increases in plasma vasopressin concentrations have been correlated with changes
in intrathoracic pressure and transmural right atrial pressure.[97]
Mechanical stimulation of peritoneal receptors also results in increased vasopressin
release,[108]
systemic vascular resistance, and
arterial pressure.[109]
However, whether increasing
IAP to 14 mm Hg is sufficient to stimulate these receptors is unknown. The increase
in systemic vascular resistance also explains why the arterial pressure increases,
whereas the cardiac output falls.[72]
[73]
[75]
Use of α2
-adrenergic agonists,
such as clonidine or dexmedetomidine,[88]
[110]
[111]
[112]
and
of
β-blocking agents[113]
significantly reduces
hemodynamic changes and anesthetic requirements. Use of high doses of remifentanil
almost completely prevents the hemodynamic changes.[81]
 |
 Figure 57-5
Schematic representation of the different mechanisms
leading to decreased cardiac output during pneumoperitoneum for laparoscopy.
Figure 57-5
Schematic representation of the different mechanisms
leading to decreased cardiac output during pneumoperitoneum for laparoscopy.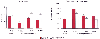 Figure 57-6
Changes in the cardiac index and systemic vascular resistance
during laparoscopy in two groups of patients. For group 1 (controls, n = 10, filled
bars), pneumoperitoneum was induced with patients in a 10-degree head-up
position. Group 2 (volume loaded, n = 10, open bars)
patients received 500 mL of lactated Ringer's solution before anesthesia induction
and were insufflated in the supine position. Data are presented as the mean ±
SEM.
Figure 57-6
Changes in the cardiac index and systemic vascular resistance
during laparoscopy in two groups of patients. For group 1 (controls, n = 10, filled
bars), pneumoperitoneum was induced with patients in a 10-degree head-up
position. Group 2 (volume loaded, n = 10, open bars)
patients received 500 mL of lactated Ringer's solution before anesthesia induction
and were insufflated in the supine position. Data are presented as the mean ±
SEM.