|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pancreas transplantation is a lengthy procedure that requires continuous attention to detail ( Fig. 56-3 ). The usual exposure requires a generous midline incision extending from the epigastrium to the symphysis pubis. The pancreas is placed in an upper abdominal location with an arterial blood supply from the aorta via a vessel graft. Several options are available for venous drainage and implantation of the exocrine duct. Venous outflow from the pancreas, which is the source of insulin, may go into the inferior vena cava and directly into the systemic circulation, or it may be directed in a more physiologically correct manner, which is into the portal vein draining the splanchnic circulation. The exocrine pancreatic flow may be connected to the small bowel or through a cuff of donor intestine into the bladder. The latter method allows for monitoring of urine amylase as a marker of rejection or injury to the transplanted pancreas. Enteric drainage is preferred for SPK because it is more physiologic and avoids bladder complications. Bladder drainage, however, appears to be a better option for PTA and PAK because of the greater likelihood of rejection with these types of transplantation. Surveillance of urine amylase allows for early detection of rejection.
Diabetic patients have a greater incidence of autonomic neuropathy, which can be manifested as both a higher heart rate and higher blood pressure than in nondiabetic ESRD patients.[101] Type 2 diabetics often have metabolic syndrome, a combination of visceral obesity, atherogenic dyslipidemia (low levels of high-density lipoprotein and elevated levels of triglycerides), hypertension, and insulin resistance. This combination leads to a heightened risk for CAD and cardiovascular disease. It should be determined when antiglycemic agents were last taken. Orally administered drugs should not be taken the day of surgery to avoid the potential for unrecognized hypoglycemia under anesthesia. Insulin-dependent patients who are extremely brittle and have declining insulin levels are at risk for ketosis and intraoperative acidemia.
As noted earlier, diabetic patients, even those without renal dysfunction, have significant risk factors for major cardiovascular complications during major surgery. Historically, the principal candidates for pancreas transplantation have been younger than those receiving
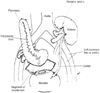 Figure 56-3
Transplantation of the pancreas with drainage of the
bladder through a pancreaticoduodenocystostomy. A renal transplant is also shown
with the common iliac vessels used for vascular anastomoses. (Modified from
Moody FG [ed]: Surgical Treatment of Digestive Disease, 2nd ed. St Louis, Mosby-Year
Book, 1990.)
Figure 56-3
Transplantation of the pancreas with drainage of the
bladder through a pancreaticoduodenocystostomy. A renal transplant is also shown
with the common iliac vessels used for vascular anastomoses. (Modified from
Moody FG [ed]: Surgical Treatment of Digestive Disease, 2nd ed. St Louis, Mosby-Year
Book, 1990.)
It was suggested in the early 1990s that diabetic patients as a group should be considered more difficult to tracheally intubate because of changes in their upper airway tissues from exposure to high serum glucose levels. In fact, one study found a 31% incidence of difficult intubation conditions in this patient population.[151] Subsequently, a large study by the Mayo Clinic reviewed the anesthetic records of 150 patients with diabetes who underwent general anesthesia and tracheal intubation and found only a slightly increased incidence of "more difficulty visualizing" airway structures.[152] Thus, it appears that long-standing DM alone does not strongly predispose to airway difficulties but may be considered a contributing factor in a patient with other potentially problematic airway features.
Because these operations are long and surgically tedious and involve extensive abdominal exposure, general endotracheal anesthesia with muscle relaxation is the best choice for these cases. Patients may have significant postoperative pain from the extensive abdominal dissection, so placement of an epidural catheter for postoperative pain control may be warranted. However, splanchnic perfusion to the transplanted organs is a major concern, and therefore some centers opt to defer placement of an epidural catheter.
Because the pancreas seems to be a highly immunogenic organ, intensive immunosuppression is needed to prevent graft loss. Administration of the initial dose of immunosuppressant will fall to the anesthesia team, so it is essential that the desired drugs be present and administered correctly in the operating room. Likewise, correct administration of the preoperative and intraoperative antibiotics requested by the surgical team is very important. Prophylaxis against enteric organisms introduced with transplanted bowel segments is crucial given the intensive immunosuppression.
Standard monitors (five-lead ECG, noninvasive blood pressure measurement, pulse oximetry, end-tidal gas concentrations) plus arterial and central venous lines are required. The arterial line allows for careful tracking of blood pressure, as well as the ability to draw samples for blood gas, glucose, and electrolyte determinations during these lengthy cases. Insertion of a central venous catheter will permit monitoring of cardiac filling pressure and central administration of drugs.
Diabetic patients, by virtue of their high incidence of autonomic dysfunction, are likely to have gastroparesis and large residual gastric volumes. This risk is even greater if the patient has ESRD and uremia. Administration of a nonparticulate antacid along with maintenance of cricoid pressure during rapid-sequence induction is mandatory.
Patients with autonomic neuropathy may be thought to be at greater risk for severe cardiovascular depression during induction of anesthesia. However, a study of uremic patients undergoing kidney transplantation found that diabetic patients with known preexisting autonomic neuropathy had hemodynamic responses to induction that were similar to those of nondiabetic uremic patients.[153] Hemodynamic stability for long periods is probably best achieved by using a balanced anesthetic technique. As with kidney transplantation alone, adequate blood pressure is desired to provide good perfusion pressure for a newly anastomosed pancreas.
One of the most challenging aspects of these cases is determining the amount and type of fluids to administer. On one hand, a lengthy intra-abdominal procedure would indicate the need to administer significant amounts of fluid to provide for large insensible and third-space losses. Administration of blood products is indicated to provide adequate oxygen-carrying capacity, platelet numbers, or clotting factors if coagulation appears to be impaired clinically and is confirmed by laboratory analysis. Patients with ESRD may have significant hypervolemia, may be hypertensive, and may display hemodynamic pathology such as diastolic dysfunction, which may limit the need or ability to give fluids. Therefore, fluid administration must be guided by cardiac preload, as indicated by chamber filling pressures, or by volume status, as determined by TEE, for example. If fluid does need to be given, it is probably best from a surgical standpoint to use colloid-based fluid rather than a large volume of crystalloid solutions alone. Although no controlled studies have been conducted, swelling of the pancreas graft appears to be less with colloids than with crystalloids.
Abdominal muscle relaxation is essential for these extensive intra-abdominal procedures. The same issues outlined previously for patients undergoing kidney transplantation apply to the choice of muscle relaxant to be used in patients undergoing SPK. Because of the long duration of these cases, a continuous infusion of cisatracurium will allow for titration of the level of block with reliable reversibility. Alternatively, the intermittent administration of vecuronium, if titrated by train-of-four monitoring, [114] can produce excellent relaxation conditions. For patients who are undergoing PTA or PAK and have adequate renal function, the use of any intermediate-acting nondepolarizing muscle relaxant should not cause problems.
Control of blood sugar intraoperatively is important to prevent the development of ketoacidosis in patients with unopposed counter-regulatory hormone secretion and allow assessment of the function of the transplanted pancreas. Before unclamping the pancreas, glucose should be checked hourly. Hyperglycemia may cause depressed immune function and decreased wound healing and put patients at an increased risk for worsened neurologic injury should brain ischemia occur.[154] After the pancreas is unclamped, glucose should be monitored every 30 minutes. Typically, glucose concentrations decrease approximately 50 mg/dL/hr after unclamping the pancreas.
A randomized trial of insulin administration to insulin-dependent type 2 diabetics compared a continuous glucose-insulin infusion with intermittent intravenous insulin injection in both major and minor surgery. Very little difference was found in the ability to control intraoperative and postoperative glucose levels and metabolism.[155] Therefore, the manner of lowering glucose does not appear to be as critical as the glucose level itself.
Successful transplantation usually results in rapidly declining insulin requirements. Blood glucose levels should be monitored closely in the recovery room or ICU to avoid hypoglycemia. If simultaneous kidney transplantation is also performed, close monitoring of urine output to detect reversible graft impingement is also necessary. Postoperative pain control can be managed with epidural or patient-controlled analgesia.
Surgical complications after these complex operations are frequent and often require one or more re-explorations during the perioperative period. If pancreas graft function is good, blood glucose levels will be corrected within days of transplantation. During the perioperative period, the same cardiac concerns that were present before transplantation will apply. However, after a period of normalized blood glucose levels and lipid profiles combined with improved renal function, the risk of major cardiac events will probably decline when compared with the preoperative risk.
|
|
|
|
|
|
|
|
|
|
|
|
|