|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Donor kidneys, whether from a living or a cadaveric source, are usually implanted in the iliac fossa. Either side of the abdomen can be used. An incision approximately 8 to 10 inches (20 to 25 cm) provides sufficient exposure. Frequently, the incision extends from the midline of the public symphysis to a point 2 to 3 cm superomedial to the anterior superior iliac spine. Anatomic variations are common and often require repair or reconstruction of vessels or the ureter. For example, two or more renal arteries can supply the kidney, with both originating from the aorta. Although vascular anastomoses can be performed with different recipient vessels, the external iliac artery and vein are frequently used for the vascular anastomoses ( Fig. 56-2 ). Distal and proximal clamps are placed on the external iliac artery and vein, and the donor renal artery (occasionally two) and vein are connected by end-to-side anastomosis. The period of warm ischemia generally lasts between 15 and 30 minutes. After completion of the anastomoses, the clamps are released in staged fashion. The bladder is subsequently filled through a Foley catheter to facilitate implantation of the ureter directly into the bladder. The wound is closed in layers with special attention paid to urine output to identify vascular or ureteral compromise during closure.
Up to 50% of patients with ESRD will have one or more of the comorbid conditions described earlier. The presence of these comorbidities will greatly influence the approach to anesthesia chosen for these patients. Although cases
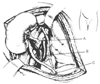 Figure 56-2
A, Renal artery anastomosis
performed end to side to the external iliac vein. B,
Renal vein anastomosis performed end to side to the external artery. C,
Ureteral anastomosis to the bladder mucosa. (From Hardy JD: Hardy's Textbook
of Surgery, 2nd ed. Philadelphia, JB Lippincott, 1988.)
Figure 56-2
A, Renal artery anastomosis
performed end to side to the external iliac vein. B,
Renal vein anastomosis performed end to side to the external artery. C,
Ureteral anastomosis to the bladder mucosa. (From Hardy JD: Hardy's Textbook
of Surgery, 2nd ed. Philadelphia, JB Lippincott, 1988.)
One method to roughly estimate volume status is to compare patients' weight with their "dry weight," which hemodialysis patients usually know. Occasionally, continuous ambulatory peritoneal dialysis is used as an alternative to hemodialysis. Patients undergoing hemodialysis may have fluid removed just before surgery to facilitate perioperative fluid management. On occasion, such removal of fluid may render patients hypovolemic and put them at risk for significant hypotension on induction of anesthesia. Potassium levels should be reviewed immediately before surgery, especially in patients who may have missed a regular dialysis appointment. Though unlikely after immediate preoperative completion of dialysis, potassium levels greater than 6.0 mEq/L may require a delay in surgery and correction of potassium levels.
Preoperative evaluation of cardiac function is of central importance and is dictated by the underlying renal disease and its duration and comorbidities. A preoperative ECG/regular echocardiogram may be sufficient for a patient who is young with newly diagnosed ESRD unrelated to diabetes, whereas a stress echocardiogram or a cardiac catheter may be indicated for a patient with long-standing ESRD associated with diabetes. Many older and
Several noninvasive screening tests have been studied to determine their ability to identify coronary artery disease (CAD) in this patient population. In a prospective study, Herzog and coworkers performed dobutamine stress echocardiography (DSE) before quantitative coronary angiography in a mixed population of ESRD patients who were candidates for transplantation.[98] More than 50% of patients had some degree of CAD (coronary stenosis >50%). However, the DSE displayed a sensitivity of only 52% to 75% and a specificity of 74% to 76% in identifying patients with CAD. These patients were monitored for up to 2 years. During this time, 20% of those with a negative DSE suffered cardiac death or myocardial infarction or underwent coronary revascularization. These authors concluded that DSE was a useful, but imperfect tool for identifying those needing further workup. However, it has to be kept in mind that DSE detects functionally significant stenosis and that most sudden cardiac deaths occur in functionally insignificant lesions (e.g., acute thrombosis in patients with coronary stenosis between 20% and 30%). Other noninvasive tests have been found to have limited use in identifying ESRD patients with CAD. In one study, dipyridamole thallium scintigraphy showed adequate sensitivity (80%) but poor specificity (37%).
More recent approaches suggest that determination of cardiac risk should begin with analyzing easily obtainable clinical variables rather than engaging in expensive tests with limited sensitivity and specificity.[99] For example, a history of chest pain is a helpful starting point in detecting CAD in these patients because it has a sensitivity/specificity of 65% for CAD.[97] A more comprehensive system called the revised cardiac risk index was originally derived from retrospective data and shown in a prospective population to be a good predictor of the cardiac risk for any patient undergoing noncardiac surgery.[100] It focuses on the presence or absence of six variables: high-risk surgical procedure, history of ischemic heart disease (excluding previous coronary revascularization), history of heart failure, history of stroke or transient ischemic attacks, preoperative insulin therapy, and preoperative creatinine levels higher than 2 mg/dL (152.5 µmol/L). One or more of these risk factors is invariably found in patients presenting for kidney transplantation. With zero or one risk factor, the rate of a major perioperative cardiac event is quite low; however, the rates rise rapidly to 6.6% and 11.0% when two or three or more of these risk factors are present. It is widely accepted that patients awaiting kidney transplantation require repeated cardiovascular surveillance. In the case of an initial negative cardiovascular evaluation, patients are risk-stratified (e.g., diabetic ESRD, nondiabetic risk factors) and assessed annually, biannually, or even less frequently. Patients with a positive initial cardiovascular workup (with or without a previous medical intervention) are mostly evaluated annually. [64]
The high cardiac risk of these patients explains the recent emphasis on the use of perioperative β-blockade as a means of decreasing perioperative risk in high-risk patients such as those undergoing kidney transplantation. A series of three studies throughout the late 1990s established that perioperative β-blockade provides significant risk protection from major cardiac events in at-risk populations. No prospective, randomized trials have been conducted with perioperative β-blockade in the kidney transplant population. It is unknown at present whether such treatment can be applied safely to these patients, especially those with DM (also see Chapter 27 ).
Diabetic patients have a higher incidence of autonomic neuropathy, which can be manifested as a higher heart rate and blood pressure than in nondiabetic ESRD patients.[101] Type 2 diabetics often have a combination of visceral obesity, atherogenic dyslipidemia (low levels of high-density lipoprotein and elevated levels of triglycerides), hypertension, and insulin resistance. This combination leads to a heightened risk of CAD and cardiovascular disease.
It should be determined when antiglycemic agents were last taken. Oral agents should not be taken the day of surgery because of the potential for unrecognized hypoglycemia under anesthesia. Insulin-dependent patients who are extremely brittle and have declining insulin levels are at risk for the development of ketosis and intraoperative acidemia.
Coagulation status, as reflected by the prothrombin time, international normalized ratio (INR), partial thromboplastin time, fibrinogen, and platelet count, is routinely assessed before surgery. Although ESRD patients may be consuming protein-restricted diets, it is rare for such diets to cause a significant clotting factor deficiency. A helpful preoperative screen to predict bleeding is a carefully conducted history that includes family, dental, obstetric, surgical, transfusion, and drug histories; the bleeding time is not a useful screening test to predict intraoperative bleeding. [102]
Although spinal anesthesia was used exclusively for the first reported anesthetics[78] and other centers have recently reported successful use of regional anesthesia for these cases,[103] [104] most centers now use general endotracheal anesthesia to provide stable hemodynamics, excellent muscle relaxation, and predictable depth of anesthesia. A balanced technique combining volatile anesthetics with opioids has been compared with total intravenous anesthesia using opioids and propofol, with no differences found.[105] Standard ASA monitors may be all that are needed for these cases. Severe cardiac arrhythmias are not common. [106] Patients with more advanced stages of comorbid conditions will require more extensive monitoring such as continuous arterial pressure or CVP monitoring, or both. Major swings in blood pressure may occur, with hypotension (49.6%) being more likely than hypertension (26.8%) in one large series.[106] Those with the most severe comorbid conditions, such as symptomatic CAD or a history of congestive heart failure, should be monitored for the development of ischemia or severe hemodynamic impairment intraoperatively with a pulmonary artery catheter or transesophageal echocardiography (TEE). The status of hemodialysis shunts or fistulas should be monitored during positioning and intraoperatively to establish the presence of a thrill and document patency.
With CVP monitoring, the goal is to keep CVP in the 10- to 15-mm Hg range to optimize cardiac output and renal blood flow. Immediate graft function has been associated with a blood volume greater than 70 mL/kg and a plasma volume greater than 45 mL/kg.[107] Another study demonstrated that pulmonary artery pressure (PAP) correlated with graft function. Higher filling pressure (PAP >20/diastolic PAP >15) resulted in better graft function than lower filling pressure did.[108] Fluid status can be modified by infusion of either crystalloid or colloid. Most recently, there has been some discussion of whether any particular crystalloid is better than the others in terms of postoperative renal function. However, no large prospective study has been published. Although the recipient's blood should be typed and screened, it is unlikely that transfusion will be necessary because blood loss is usually minimal.
Patients with uremia and other comorbid conditions (e.g., diabetes) should be considered at risk for aspiration during induction of anesthesia. Pretreatment with a clear, nonparticulate antacid such as 30 mL of sodium citrate raises gastric pH. To prevent reflux and aspiration, rapid-sequence induction while maintaining cricoid pressure should be considered.
Several studies have demonstrated that the induction dose of propofol to achieve clinical hypnosis, as well as reduction of the bispectral (BIS) index to 50, was 40% to 60% higher than needed for normal patients.[109] [110] In the study of Goyal and colleagues,[110] 0.2 mg/kg of propofol was titrated every 15 seconds to predefined end points. The authors found a negative correlation between the propofol dose and preoperative hemoglobin levels. The exact mechanism is not entirely understood, and administering an increased bolus induction dose based on these findings is strongly discouraged.
Patients with preexisting hypertension are at risk for large fluctuations in arterial blood pressure and heart rate during induction of anesthesia and endotracheal intubation. Given that these patients have a high prevalence of CAD and myocardial ischemia, goals at induction should include careful control of the heart rate and blood pressure to minimize the possibility of myocardial ischemia. Several methods have been used to achieve adequate heart rate and blood pressure control during induction. Moderate to large doses of opioids such as fentanyl can blunt the response to laryngoscopy; however, blood pressure may frequently be more difficult to maintain without the use of vasoconstrictors after induction. In recent years, the short-acting opioid remifentanil, metabolism of which occurs in plasma, has been an effective drug for good heart rate control. Furthermore, the rate of remifentanil administration can be titrated to allow for rapid adjustment of anesthetic depth. The short-acting β-adrenergic blocker esmolol (0.5 to 1.0 mg/kg) is used to blunt the hemodynamic response to intubation and is ideally suited in this patient population with an adequate ejection fraction.
The use of succinylcholine is not contraindicated in patients with ESRD.[111] Plasma cholinesterase activity has been reported to be below normal in more than 20% of ESRD patients regardless of whether they were receiving any form of dialysis. Whether receiving peritoneal dialysis, hemodialysis, or no dialysis, they did not experience prolonged paralysis after an intubating dose of succinylcholine unless they also had an atypical form of serum cholinesterase.[112] The increase in serum potassium after an intubating dose of succinylcholine was found to be the same, approximately 0.6 mEq/L for patients with and without ESRD.[113] This increase can be tolerated without significant cardiac risk even with an initial serum K+ concentration greater than 5 mEq/L.
Drugs such as gallamine, metocurine, pancuronium, and curare depend on the kidney for elimination and thus have a prolonged duration of action in patients with ESRD. Atracurium and cisatracurium are metabolized by spontaneous Hoffman degradation and plasma cholinesterase, which makes their duration of action independent of either liver or kidney function. Patients with ESRD have been shown to have increased sensitivity to vecuronium with a prolonged duration of action.[114]
The choice of inhaled anesthetic includes desflurane, isoflurane, and sevoflurane. The metabolism of sevoflurane has been implicated in renal toxicity, but no controlled studies are available to clearly indicate either safety concerns or danger when using sevoflurane in the setting of a newly transplanted kidney. There are two elements of concern with regard to toxicity: (1) production of fluoride ion from the metabolism of sevoflurane and (2) generation of "compound A" by the breakdown of sevoflurane by sodium or barium hydroxide lime. Sevoflurane appears to have a very good safety record. It has been administered to tens of millions of patients worldwide with no conclusive evidence of renal toxicity. Two volunteer studies have found biochemical evidence of renal injury during sevoflurane anesthesia, whereas five other volunteer studies have not.[115] [116] [117] [118] [119] [120] [121] However, kidney transplantation represents a situation of increased renal risk, as recently defined by Artru.[122] Two studies have shown that sevoflurane, at fresh gas flow rates greater than 4 L/min, did not change renal function indices or biochemical markers in patients with mildly impaired renal function (baseline creatinine >1.5 mg/dL).[123] [124] [125]
With regard to perioperative pain control, drugs such as morphine, meperidine (Demerol), or oxycodone should be used with caution in renal failure patients because they or one of their active metabolites are dependent on renal excretion and thus may accumulate.[126] [127] [128] In contrast, fentanyl, sufentanil, alfentanil, and remifentanil are safe alternatives. The pharmacokinetic parameters of sufentanil are very similar in patients with ESRD and healthy control patients.[126] [129] Metabolism of alfentanil occurs by inducible cytochrome c enzymes in the liver. Remifentanil's duration of action is short, even in patients with ESRD.[130] The principal metabolite of remifentanil, GR90291, is eliminated primarily by the kidneys. The fact that it is only 1/4000 as potent as the parent compound makes remifentanil safe to use in these patients.[131]
Hypotension may occur after unclamping the iliac vessels and reperfusion of the graft. Because renal graft function is critically dependent on adequate perfusion, every effort should be made to avoid episodes of marked hypotension. It is generally thought that vasoconstrictors with strong α-adrenergic effects, such as phenylephrine, should be drugs of last resort. Several animal models have demonstrated that vessels in the transplanted organ appear to be more sensitive to sympathomimetics, which
Immediate urine production is seen in over 90% of living donor kidney transplants and between 40% and 70% of cadaveric transplants. During the latter stages of closure of the surgical wound, a decrease in urine output strongly suggests mechanical impingement of the graft, vessel, or ureter. The Foley catheter should be irrigated to ensure that clot or tissue has not affected its patency. If intraoperative ultrasound is immediately available, it can be used to examine flow in the arterial and venous anastomoses. In addition to maintaining adequate intraoperative perfusion pressure,[134] urine production is frequently enhanced intraoperatively by mannitol, loop diuretics, and occasionally dopamine. Mannitol is freely filtered and not reabsorbed by the nephron, which causes osmotic expansion of urine volume. It may also have a protective effect on the cells lining the renal tubule. It is usually administered to donors before harvesting and to recipients just before unclamping the arterial blood flow. In this way mannitol may protect from ischemic injury, as well as induce osmotic diuresis in the newly transplanted kidney. In most centers, relatively low doses of mannitol are administered, generally in the range of 0.25 to 0.5 mg/kg, and thus reports of large changes in electrolyte concentrations are unwarranted. Delayed graft function of cadaveric kidneys can be prevented by the intraoperative administration of mannitol.[135] Loop diuretics work by blocking the action of Na+ /K+ channels present in the thin ascending limb of Henle, thereby preventing reabsorption of electrolytes in this segment of the nephron. The high-osmolar fluid that is carried along to the distal tubule prevents the reabsorption of water and thus causes the excretion of large volumes of urine with high electrolyte content.
Although the main effect of loop diuretics is increased urine output, the ability to prevent oliguria (<400 mL/day) can be a significant achievement.
"Renal-dose" or low-dose dopamine (2 to 3 µg/kg/min) is commonly used to stimulate DA1 dopaminergic receptors in the kidney vasculature to induce vasodilation and increased urine output. Some small trials have shown improved urine output[136] and creatinine clearance [137] with low-dose dopamine during kidney transplantation, whereas other larger studies have shown no significant improvement.[138] [139] The utility of this approach has been questioned in that a newly transplanted, denervated kidney may not respond to low-dose dopamine like normal kidneys do. Doppler ultrasound examination of newly transplanted kidneys found no significant change in blood flow at dopamine infusion rates of 1 to 5 µg/kg/min. [140]
Another dopamine agonist, fenoldopam, has been used to maintain renal blood flow during cardiopulmonary bypass (CPB). It has also been administered during surgery involving aortic cross-clamping, as well as in critically ill patients. However, the effects of fenoldopam on the function of newly transplanted kidneys have not been systematically studied.
All renal transplant patients should have their anesthesia fully reversed, be extubated if possible, and be taken to the postoperative recovery area. In general, renal transplant patients have a low incidence of postoperative ICU admission, around 1% in one large series. If ICU admission is required, it is usually for sepsis or fluid overload.[141]
Urine output is closely monitored, and any significant decline should raise suspicion of a possibly correctable mechanical problem with the newly transplanted kidney. Re-exploration of the wound should not be delayed if kinking of the vascular attachments or obstruction of the ureter along its course or at the site of bladder reimplantation is suspected.
Postoperative pain is usually mild to moderate after kidney transplantation. One study showed that the choice of intraoperative anesthetic influenced postoperative pain control—patients who received propofol as a component of anesthesia had better recovery of psychomotor function and used patient-controlled analgesia more effectively than did those receiving halothane or isoflurane.[142] Placement of intercostal nerve blocks did not change the use of patient-controlled analgesia or pain scores after surgery.[143]
Patients with good graft function as determined by laboratory values (blood urea nitrogen, creatinine) and with sufficient urine volume should be considered to have adequate renal function. The average glomerular filtration rate (GFR) 6 months after cadaveric renal transplantation is almost 50 mL/min.[144] About 50% of patient will manifest a slow decline in GFR over the course of years, but 30% will have a stable GFR. Given the improvement in patient survival after renal transplantation, it is likely that the rapid progression of cardiovascular disease is slowed, but not halted. These patients are still at higher cardiac risk than those who did not experience ESRD. Aside from the remaining traditional cardiac risk factors, graft rejection, viral infection, anemia,[84] [145] and treatment with immunosuppressive drugs such as cyclosporine may cause significant cardiovascular morbidity (e.g., hypertension), but newer immunosuppressants appear to be better tolerated.[146] Congestive heart failure (e.g., cardiomyopathy), LV hypertrophy, and ischemic heart disease remain important complications in renal transplant recipients.[84]
|
|
|
|
|
|
|
|
|
|
|
|
|