|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The best indication that a patient has renal disease is usually obtained from the medical history. Physical findings are often minimal until renal disease is far advanced unless hypertension is present. Urinalysis is sufficient laboratory screening for identification of kidney disease if the patient does not have a history of genitourinary abnormalities. If renal disease is thought to be present, more precise methods of assessing renal function are necessary. Laboratory tests useful in evaluating renal function are described next ( Table 54-2 ).
The GFR is by far the best measure of glomerular function. Normal
GFR is around 125 mL/min. However, manifestations of reduced GFR are not seen until
the GFR has decreased to 50% of normal. The effect of impaired GFR is a reduction
in the total rate of delivery of solute into the glomerular filtrate. When accompanied
by comparably reduced rates of urinary excretion, retention and accumulation of the
unexcreted solute occur and result
| Test Name | Reference Range | Units |
|---|---|---|
| Urea nitrogen | 5–25 | mg/dL |
| Creatinine | 0.5–1.5 | mg/dL |
| Sodium | 133–147 | mmol/L |
| Potassium | 3.2–5.2 | mmol/L |
| Chloride | 94–110 | mmol/L |
| CO2 | 22–32 | mmol/L |
| Uric acid | 2.5–7.5 | mg/dL |
| Calcium | 8.5–10.5 | mg/dL |
| Phosphorus | 2.2–4.2 | mg/dL |
| Urinalysis, routine |
|
|
| Color | Straw-amber |
|
| Appearance | Clear-hazy |
|
| Protein | Zero | mg/dL |
| Blood | Negative |
|
| Glucose | Zero | mg/dL |
| Ketones | Zero | mg/dL |
| pH | 4.5–8.0 |
|
| Specific gravity | 1.002–1.030 |
|
| Bilirubin | Negative |
|
| Urinalysis, micro |
|
|
| Red blood cells | 0 to 3 | per High-power field |
| White blood cells | 0 to 5 | per High-power field |
| Casts | 0 to 2 | per Low-power field |
| From Miller ED Jr: Understanding renal function and its preoperative evaluation. In Malhotra V (ed): Anesthesia for Renal and Genitourinary Surgery. New York, McGraw-Hill, 1996, p 9. | ||

The blood urea nitrogen (BUN) concentration is not a direct correlate of reduced GFR. BUN is influenced by nonrenal variables such as exercise, bleeding, steroids, and massive tissue breakdown. The more important factor is that BUN is not elevated in kidney disease until the GFR is reduced to almost 75% of normal. [5]
Measurements of creatinine provide valuable information regarding general kidney function. Creatinine in serum results from turnover of muscle tissue and is dependent on daily dietary intake of protein; normal values are in the range of 0.5 to 1.5 mg/100 mL, with values of 0.5 to 1.0 mg/100 mL present during pregnancy. Creatinine is freely filtered at the glomerulus, and apart from an almost negligible increase in content because of secretion in the distal nephron, it is neither reabsorbed nor secreted. Therefore, serum creatinine measurements reflect glomerular function ( Fig. 54-4 ),[5] and creatinine clearance is a specific measure of GFR. Because there is such a wide range in normal values, a 50% increase in serum creatinine concentration, indicative of a 50% reduction in GFR, may go undetected unless baseline values are known. It should also be apparent that excretion of drugs dependent on glomerular filtration may be significantly decreased despite what might seem to be only slightly elevated serum creatinine values (1.5 to 2.5 mg/100 mL). The serum creatinine concentration and clearance are better indicators of general kidney function and GFR than similar measurements of urea nitrogen are. Urea nitrogen concentration and clearance are subject to wide intraindividual variations secondary to changes in hydration, rate of urine flow, and dietary protein intake.
Creatinine clearance is measured over a 24-hour period and is
calculated as follows:
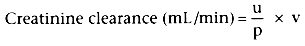
where u = urinary concentration of creatinine (mg/100 mL)
p = plasma concentration of creatinine (mg/100 mL)
v = urine volume (mL/min) A 24-hour clearance is more accurate than a 2-hour creatinine
clearance test, which is frequently used because it is more convenient. Normal values
are 85 to 125 mL/min in
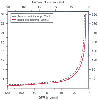 Figure 54-4
Theoretical relationship between blood urea nitrogen
(BUN) and creatinine versus the glomerular filtration rate (GFR). (Redrawn
from Kassirer JP: Clinical evaluation of kidney function-glomerular function. N
Engl J Med 285:385, 1971.)
Figure 54-4
Theoretical relationship between blood urea nitrogen
(BUN) and creatinine versus the glomerular filtration rate (GFR). (Redrawn
from Kassirer JP: Clinical evaluation of kidney function-glomerular function. N
Engl J Med 285:385, 1971.)
Urinary specific gravity is an index of the kidney's concentrating ability, specifically, renal tubular function. Determination of urinary osmolality, that is, measurement of the number of moles of solute (osmoles) per kilogram of solvent, is a similar, more specific test. Excretion of concentrated urine (specific gravity, 1.030; 1050 mOsm/kg) is indicative of excellent tubular function, whereas a urinary osmolality fixed at that of plasma (specific gravity, 1.010; 290 mOsm/kg) is indicative of renal disease. The urinary dilution mechanism persists after concentrating defects are present, so a urinary osmolality of 50 to 100 mOsm/kg may still be consistent with advanced renal disease.
Patients without renal disease may excrete up to 150 mg of protein per day; greater amounts may be present after strenuous exercise or after standing for several hours. Massive proteinuria (i.e., >750 mg/day) is always abnormal and is usually indicative of severe glomerular damage. However, proteinuria also may be due to (1) failure of tubular reabsorption of the small amount of protein that is normally filtered, (2) abnormally increased concentrations of normal plasma proteins, or (3) the presence of abnormal plasma proteins, which are then excreted in urine.
Glucose is freely filtered at the glomerulus and is subsequently reabsorbed in the proximal tubule. Glycosuria signifies that the ability of the renal tubules to reabsorb glucose has been exceeded by an abnormally heavy glucose load and is usually indicative of diabetes mellitus. However, glycosuria may also be present in hospitalized patients without diabetes who are receiving intravenous glucose infusions.
Gross and microscopic observation of urine and its sediment, along with determination of urinary pH, specific gravity, protein content, and sugar content, is one of the most readily available, inexpensive, and informative laboratory tests. The gross appearance of urine may indicate the presence of bleeding or infection in the genitourinary tract. Microscopic examination of urinary sediment may reveal casts, bacteria, and various cell forms, thereby supplying diagnostic information in patients with renal disease.
Urinary pH is a measure of the ability of the kidneys to acidify urine. The kidneys share regulation of acid-base balance with the lungs and provide the sole pathway of excretion for the 60 mEq of hydrogen ion (nonvolatile acid) produced each day by normal metabolism.[6] The three renal mechanisms that prevent the development of acidemia are reabsorption of filtered bicarbonate, acidification of buffers in tubular urine (i.e., excretion of titratable acid), and production of ammonia in tubular cells and its excretion as ammonium ion. The inability to excrete an acidic urine in the presence of systemic acidosis is indicative of renal insufficiency.
Anemia may be present in patients with renal disease because of abnormalities in the production of erythropoietin (erythropoiesis-stimulating factor [ESF].) The exact mechanism of ESF formation is unknown.[7] [8] [9] One view is that in response to hypoxia, the kidney elaborates a precursor of ESF that combines with a plasma protein to form active ESF.[7] Another theory is that the kidney produces an enzyme, renal erythropoietic factor, that converts a precursor in plasma to ESF.[7] Patients with advanced renal disease do not appear to have decreased ESF activity and hence anemia. [8] The absence of ESF, as may occur in an anephric patient, results in hemoglobin levels of 6 to 8 g/100 mL. A recently available commercial preparation of erythropoietin has been effective in alleviating the chronic anemia associated with end-stage renal disease (ESRD). Hemoglobin concentrations of 10 g/100 mL or greater are not uncommon in patients with ESRD treated with recombinant erythropoietin. White blood cell and platelet counts are of particular importance in patients who have a transplanted kidney because immunosuppressive therapy may cause bone marrow suppression.
Sodium, potassium, chloride, and bicarbonate concentrations should be determined if impairment in renal function is suspected. However, the results of these tests usually remain normal until frank renal failure is present, and hyperkalemia does not occur until patients are uremic.[10] Measuring urinary sodium or chloride excretion is especially useful when attempting to differentiate between causes of hyponatremia, as seen in volume contraction (whether a decrease in total circulatory volume or a decrease in effective arterial blood volume), versus conditions associated with increased salt loss, such as the syndrome of inappropriate antidiuretic hormone secretion, salt-losing nephropathy, or adrenal insufficiency. [11]
If significant renal disease is present, patients consuming a diet high in animal protein may have metabolic acidosis.
The electrocardiogram (ECG) (see Chapter 34 ) reflects the toxic effects of potassium excess more closely than determination of the serum potassium concentration does. The serious effect of hyperkalemia is cardiac toxicity, which does not correlate well with the plasma K+ concentration. The earliest ECG changes include increased T-wave amplitude, or peaked T waves. This finding is seen in only 25% of patients. More severe degrees of hyperkalemia result in a prolonged PR interval and QRS duration, atrioventricular conduction delay, and loss of P waves. Progressive widening of the QRS complex and merging with the T wave produce a sine wave pattern. The terminal event is usually ventricular fibrillation or asystole. Cardiac conduction abnormalities are seen when the K+ concentration equals 7.0 mEq/L.[12]
A radiograph of the kidney, ureters, and bladder is usually helpful as the initial diagnostic study to locate renal stones. Ultrasonography[13] can be used to rapidly determine whether hydronephrosis exists and to differentiate between benign and malignant cysts. Intravenous pyelography is used to evaluate anatomic features of the renal excretory system.
Radiocontrast nephropathy can occur in patients with chronic renal failure (CRF), diabetes mellitus, or multiple myeloma. A frequency as high as 50% has been reported in some patients receiving high doses of radiocontrast material for cardiac catheterization, but renal failure secondary to intravenous pyelography is relatively rare if the patient is adequately volume expanded. Computed tomography and magnetic resonance imaging are other diagnostic tools that better define the anatomy, size, and extent of tumors and masses.
Radionuclide activity can be measured as counts per volume of fluid by appropriate counters or by the number of energy emissions detected by a gamma camera (α- and β-emitters do not have long enough penetration to be of use clinically). The principal use of the former method is to measure GFR and renal blood flow, whereas the latter technology is used for imaging and estimating relative renal function.
The earliest stage of CRF is a phase of reduced renal reserve. When renal function is normal, the GFR usually increases by 30% in response to the stimulus of a protein challenge. In the first stage the response to a protein challenge is attenuated. This early stage is particularly well documented in diabetic nephropathy. At this stage, determination of GFR is helpful in diagnosis.
As the GFR decreases to 10% to 30% of normal, a stage of moderate renal insufficiency sets in. Patients remain asymptomatic with only biochemical evidence of a decline in GFR (i.e., an increase in serum concentrations of urea and creatinine). Further workup usually reveals other abnormalities such as nocturia, anemia, loss of energy, decreasing appetite, and abnormalities in calcium and phosphorus metabolism.
As the GFR decreases further (30% of normal), a stage of severe
renal insufficiency begins. This stage is characterized by profound clinical manifestations
of uremia and biochemical abnormalities such as acidemia, volume overload, and neurologic,
cardiac, and respiratory manifestations. At the stages of mild and moderate renal
insufficiency, intercurrent clinical stress may compromise renal function even further
and induce signs and symptoms of overt uremia. When the GFR is 5% to 10% of normal,
it is called ESRD, and continued survival without renal replacement therapy becomes
impossible. Although
| Improved by Dialysis | Improved by Adding Erythropoietin | Variable Response | Not Improved | Develop after Dialysis Therapy |
|---|---|---|---|---|
| Volume expansion and contraction | Fatigue | Secondary hyperparathyroidism | Increased lipoprotein level | Adynamic osteomalacia |
|
|
Impaired mentation |
|
|
β2 -Microglobulinemia |
| Hypernatremia and hyponatremia | Lethargy | Hyperuricemia | Decreased high-density lipoprotein level | Muscle cramps |
|
|
Pallor | Hypertriglyceridemia |
|
Dialysis disequilibrium syndrome |
| Hyperkalemia and hypokalemia | Anemia | Protein-calorie malnutrition |
|
|
|
|
Bleeding diathesis |
|
Impaired growth and development | Hypotension and arrhythmias |
| Metabolic acidosis |
|
Headache |
|
|
| Hyperphosphatemia |
|
Peripheral neuropathy | Infertility and sexual dysfunction | Hepatitis |
| Hypocalcemia |
|
Restless legs syndrome |
|
Idiopathic ascites |
| Vitamin D-deficient osteomalacia |
|
Paralysis | Amenorrhea | Peritonitis |
|
|
|
Seizures | Amenorrhea | Leukopenia |
| Carbohydrate intolerance |
|
Myopathy | Sleep disorders | Hypocomplementemia |
| Hypothermia |
|
Arterial hypertension | Pruritus |
|
| Asterixis |
|
Cardiomyopathy | Lymphocytopenia |
|
| Muscular irritability |
|
Accelerated atherosclerosis | Splenomegaly and hypersplenism |
|
| Myoclonus |
|
|
|
|
| Coma |
|
Vascular calcification |
|
|
| Congestive heart failure or pulmonary edema |
|
Hyperpigmentation |
|
|
|
|
|
Peptic ulcer |
|
|
| Pericarditis |
|
Gastrointestinal bleeding |
|
|
| Uremic lung |
|
Increased susceptibility to infection |
|
|
| Ecchymoses |
|
|
|
|
| Uremic frost |
|
|
|
|
| Anorexia |
|
|
|
|
| Nausea and vomiting |
|
|
|
|
| Uremic fetor |
|
|
|
|
| Gastroenteritis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|