|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although perioperative management for thoracic organ transplantation is considered elsewhere in this text, the application of these procedures to children requires some specific modification. Differences include the characteristics of the candidates, preparation of these children, anesthetic management, surgical considerations, post-bypass management, and outcome.
Even though some of the earliest heart transplants were performed for congenital heart malformations, this indication became rare by the early 1980s. In 1984, over 60% of the few pediatric heart transplants were performed in patients with cardiomyopathy, usually adolescents. In the next decade, a dramatic rise in the number of infants and young children with congenital heart malformations treated with heart transplantation resulted in a marked shift in the demographics ( Fig. 51-16 ).[240] By 1995, over 70% of the children receiving heart transplants were
Children considered for heart transplantation are more likely to have pulmonary hypertension than adults. Most adult transplant programs will not offer heart transplant therapy to patients with PVR over 6 Wood units/m2 . [241] The exclusion threshold in infants and children remains controversial. Some programs accept patients with PVR as high as 12 Wood units/m2 , particularly if the pulmonary vasculature responds to vasodilators such as oxygen, NO, calcium channel blockers, or prostacycline.[242] Neonates are generally assumed to have elevated PVR, but outcome data from some programs suggest that the importance of this factor on postoperative outcome is substantially less in the first year of life, perhaps because the infant donor hearts, having recently undergone transitional circulation, are better prepared to cope with the right ventricular pressure load that elevated PVR imposes.[243]
The anesthetic plan for pediatric heart transplantation must accommodate a wide spectrum of pathophysiology. Recipients with congenital heart malformations benefit from the analysis of loading conditions and optimizing hemodynamics discussed previously. Although a few of these patients undergo heart transplantation because the natural history of reconstructive heart surgery poses greater risk despite reasonable ventricular function, most candidates exhibit some manifestations of impaired ventricular performance. As such, they require careful titration of anesthetic agents with minimal myocardial depressant characteristics to avoid cardiovascular collapse. In this
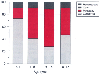 Figure 51-17
Indication for heart transplantation in children. Over
the past two decades, the major indications for pediatric heart transplantation were
nearly equally divided between congenital malformation and cardiomyopathy. In later
years, pediatric recipients with congenital malformations assumed a slight plurality
due to shifting age demographics. As illustrated, younger children are more likely
to undergo heart transplant because of congenital malformation. (Data from
the Registry of the International Society for Heart and Lung Transplantation.[240]
)
Figure 51-17
Indication for heart transplantation in children. Over
the past two decades, the major indications for pediatric heart transplantation were
nearly equally divided between congenital malformation and cardiomyopathy. In later
years, pediatric recipients with congenital malformations assumed a slight plurality
due to shifting age demographics. As illustrated, younger children are more likely
to undergo heart transplant because of congenital malformation. (Data from
the Registry of the International Society for Heart and Lung Transplantation.[240]
)
Although orthotopic heart transplantation poses some technical challenges in neonates and young infants, the replacement of an anatomically normal heart is less complex than several reconstructive heart procedures commonly performed in patients at this age. However, the need to adapt this procedure to incorporate repair of major concurrent cardiovascular malformations requires the consummate skill and creativity that remain the province of a few exemplary congenital heart surgeons. [244] [245]
Having withstood extended ischemic periods, heart grafts are extraordinarily intolerant of superimposed residual hemodynamic loads that might accompany imperfect vascular reconstruction. The extensive vascular repair and, particularly in older children with longstanding hypoxemia, the propensity to coagulopathy together elevate hemorrhage to a major cause of morbidity and even mortality in pediatric heart transplantation. Nevertheless, once successfully implanted, these grafts will respond to physiologic factors that stimulate growth and adaptation in the developing infant and child. [246]
Management considerations during separation from CPB and the early postoperative period are primarily focused on three pathophysiologic conditions: myocardial preservation, denervation, and PVR. Even expeditious transplants usually force the heart to endure ischemic periods that exceed those encountered during reconstructive surgery. Although some centers believe the infant heart is more tolerant of extended ischemia,[243] these hearts will demonstrate a period of reperfusion injury, and virtually all require pharmacologic, and in some cases mechanical, support. In addition, endogenous adaptive responses and exogenous pharmacologic agents that act via myocardial sympathetic activation are ineffective in the denervated graft. Since the majority of children presenting for heart transplantation exhibit some element of elevated PVR, even with isolated end-stage cardiomyopathy, the RV of a newly implanted heart is particularly vulnerable to failure.
As such, ventilatory and pharmacologic interventions are usually configured to exert a favorable impact on PVR and provide inotropic and chronotropic support. Once the lungs are fully expanded, we ventilate to PaCO2 values in the low 30s using an FIO2 of 1. Virtually all recipients receive low-dose dopamine (3–5 µg/kg/min) and isoproterenol (0.02–0.05 µg/kg/min) to promote inotropy, chronotropy, and lower PVR. In the event that these do not provide sufficient inotropy in the face of more significant postischemic dysfunction, additional agents are added (e.g., milrinone, epinephrine). Most transplant centers have a specific regimen for immunosuppression to be initiated in the perioperative period. As with adults, pediatric transplant programs typically employ triple drug
National statistics indicate that the outcome from pediatric heart transplantation is slightly less favorable than comparable adult results.[240] The principal risk factors are age under 1 year and congenital heart defects. Since these factors are closely related (i.e., the vast majority of infants under 1 undergo transplantation for congenital heart defect), it is difficult to determine the independent effect of age. Concurrent repair of structural cardiovascular anomalies substantially increases perioperative risk of hemorrhage, residual hemodynamic loading conditions, and right heart failure from elevated PVR. Taken together, infants under 1 year of age have an operative mortality rate of 24%, more than twice that of older children. [240] Beyond the early postoperative period, mortality rates are quite comparable for all age groups. Nevertheless, the sequelae of rejection and the consequences of the requisite immunosuppression result in significant ongoing morbidity and mortality. Because even the best transplant recipients have achieved only a 28% 14-year survival rate, these procedures must be considered palliative for children.[240]
Lung and heart-lung transplantation have achieved respectable operative survival rates in children.[249] [250] They remain the only viable surgical therapy for infants and children with severe pulmonary vascular disease and selected progressive pulmonary diseases. These remain uncommon procedures in pediatrics. Lung transplantation carries the additional morbidity of obliterative bronchiolitis, a debilitating small airway disease that results in gradual deterioration in flow-related pulmonary functions over time. Despite an operative mortality rate that is currently less than 20%, the 3-year survival rate is only 50% to 60%.[240] [250]
|
|
|
|
|
|
|
|
|
|
|
|
|