|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Modern pediatric cardiac anesthesia must include the principles and practice of effective anticoagulation, hemostasis, and blood conservation. Bleeding after CPB remains a significant problem in pediatric cardiac surgery.[217] Continuing blood loss post-CPB requiring blood component replacement is associated with hemodynamic compromise as well as morbidity from multiple donor exposures. In pediatric patients, restoration of hemostasis has proved difficult; diagnosis of the problem and treatment are marginally effective.
Neonates, infants, and children undergoing cardiac surgery with CPB have a higher rate of postoperative bleeding than that seen in older patients. [217] This is due to several factors. First, there is disproportionate exposure to the nonendothelialized extracorporeal circuit, which produces an inflammatory-like response.[17] This inflammatory response to CPB is inversely related to patient age; the younger the patient, the more pronounced the response.[17] [18] Because complement and platelet activation are linked to the activation of other protein systems in the blood (i.e., fibrinolytic), it is probable that this hemostatic activation, which results in impaired hemostasis and increased bleeding tendency, plays a major role during pediatric cardiac surgery. Second, the type of operation performed in neonates and infants usually involves more extensive reconstruction and suture lines, creating more opportunities for surgical bleeding than in adult cardiac patients. Operations are also frequently performed using deep hypothermia or circulatory arrest, which may further impair hemostasis.[218] Third, the immature coagulation system in neonates may also contribute to impaired hemostasis.[219] Although procoagulant and factor levels may be reduced in young patients with congenital heart disease due to immature or impaired hepatosynthesis,[220] functional bleeding tendencies are usually not present before surgery. Finally, patients with cyanotic heart disease demonstrate an increased bleeding tendency before and after CPB.[221]
CPB is a significant thrombogenic stimulus requiring anticoagulation with heparin prior to its initiation. Heparin is usually administered empirically based on patient weight, and its effect is followed by activated clotting time monitoring. Because the heparin effect is primarily due to coupling with antithrombin III and because there are age-related differences and quantitative differences in procoagulants and inhibitors, variability of heparin dosing and its effect has been a concern. High heparin sensitivity is observed in the 1st week of life and then decreases progressively until about 3 years of age, when values approach those observed in adults.[222] These findings are consistent with evidence in infants of variable quantities of both procoagulants and inhibitors, especially prothrombin and antithrombin III.[223]
Heparin is neutralized with protamine dosed according to the quantity of heparin administered or based on body weight. Protamine excess may actually contribute to postoperative bleeding.[224] It appears that the protamine dose requirement is high for neonates and decreases with increasing age of the patient. The relatively increased protamine requirement for young as compared with older children and adults is indicative of higher circulating heparin levels after CPB.[225] Delayed hepatic clearance of heparin due to organ immaturity and the predominant use of hypothermic circulatory arrest in the young will decrease metabolism and excretion of heparin. We typically administer 4 mg/kg protamine in neonates, whereas 2 mg/kg usually restores the activated clotting time to baseline values in adolescents and adults. Interpatient variability mandates some form of individual assessment to guide drug dose, in order to prevent excess protamine.[224]
Neonates and young infants with congenital heart disease will have low circulating levels of procoagulants and inhibitors. The thrombogenic and dilutional effects of CPB further contribute to hemostatic abnormalities after CPB. Formed blood elements such as leukocytes and platelets may be activated, and procoagulants may be diluted by CPB. Furthermore, deep hypothermic circulatory arrest causes increased clotting and fibrinolytic activity. The lower the temperature, the higher the degree of hemostatic activation. Therefore, the causes of bleeding post-CPB are many. Injudicious use of blood products to correct individual coagulation abnormalities separately can further exacerbate dilution of existing procoagulants as well as carry the risks of multiple donor exposure. Because the transfusion of blood products is associated with numerous complications, transfusion is to be assiduously avoided, unless specifically indicated by an impairment in tissue oxygenation or documented coagulopathies with clinically significant bleeding. All efforts at blood conservation during cardiac surgery should be routinely employed by all members of the operative team, intraoperatively as well as postoperatively.
Bleeding after CPB is not an unusual occurrence. The surgeon should first attempt to identify any obvious source of surgical bleeding at the sites of repair. Next, adequate protamine reversal of heparin is assessed by measuring an activated clotting time. In general, standard coagulation tests show a prolongation of the partial thromboplastin time, prothrombin time, hypofibrinogenemia, and dilution of other procoagulants as well as a prolonged bleeding time in many pediatric patients, with and without bleeding ( Fig. 51-15 ). The most common reason for persistent bleeding is platelet dysfunction.[226] [227] Under such circumstances, administration of platelets is warranted in the presence of bleeding. Routine administration of blood products to correct laboratory coagulation abnormalities in the absence of bleeding is never clinically indicated. After platelets have been given and if
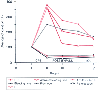 Figure 51-15
Plot of blood coagulation profile changes before, during,
and after cardiopulmonary bypass (CPB) in 25 children. Clotting times and coagulant
factors are shown as percent change from control. Stage I, baseline, before CPB;
stage II, post-CPB, before protamine reversal of heparin; stage III, after protamine;
stage IV, just prior to leaving the operating room; stage V, after 3 hours in the
intensive care unit (ICU). PTT, partial thromboplastin time; PT, prothrombin time.
Figure 51-15
Plot of blood coagulation profile changes before, during,
and after cardiopulmonary bypass (CPB) in 25 children. Clotting times and coagulant
factors are shown as percent change from control. Stage I, baseline, before CPB;
stage II, post-CPB, before protamine reversal of heparin; stage III, after protamine;
stage IV, just prior to leaving the operating room; stage V, after 3 hours in the
intensive care unit (ICU). PTT, partial thromboplastin time; PT, prothrombin time.
Many attempts have been made to reduce bleeding after CPB by pharmacologic interventions. Desmopressin acetate[228] [229] and the antifibrinolytics aminocaproic acid and tranexamic acid[230] have been tried with variable success in significantly reducing postoperative blood loss after cardiac surgery. However, the most impressive results have been demonstrated with the use of aprotinin.[231] A proteinase inhibitor, aprotinin has antifibrinolytic properties in low concentrations and acts as a kallikrein inhibitor at higher levels. CPB causes increased kallikrein by contact activation, promoting thrombus and fibrin generation, which promotes fibrinolysis. The inhibition of kallikrein results in inhibition of the contact phase of coagulation, and the inhibition of fibrinolysis reduces bleeding. Reduced thrombin generation leads to a diminished platelet stimulation. Better preserved
Although several studies have demonstrated efficacy in reducing hemorrhage and donor exposures after congenital heart surgery, this benefit may not be evident with simple primary cardiac repairs (e.g., VSD, ASD, tetralogy of Fallot). [237] [238] To date, the use of aprotinin for congenital heart surgery has been generally confined to certain patients requiring re-operation, patients undergoing a Ross procedure, and in cardiac/lung transplantation procedures. The dose of aprotinin used by us consists of a 1-ml test dose when aortic and venous purse strings are placed and cannulation is feasible. This test dose is followed by 3 mL/kg intravenously prior to the initiation of CPB and an additional 3 mL/kg in the pump prime. The risk of anaphylaxis with re-exposure to aprotinin is especially great in the first 6 months after first exposure.
The techniques of blood conservation must be continued during the postoperative period. Isolated coagulation abnormalities are often present in the uncomplicated postoperative cardiac patient (see Fig. 51-15 ). Usually, these coagulation abnormalities self-correct during the first postoperative day and are not associated with excessive bleeding. Therefore, routine correction of these abnormalities with infusion of blood products is not warranted. Administration of blood products should not occur in the absence of clinical evidence of bleeding and the identification of a specific defect requiring targeted component therapy. Routine use of blood products for volume replacement is also to be avoided; lactated Ringer's or saline solution can be satisfactorily administered at a reduced cost without the hazards associated with transfusion.
|
|
|
|
|
|
|
|
|
|
|
|
|