CARDIOPULMONARY BYPASS
CPB Circuitry
By definition, CPB diverts blood away from both the left and right
sides of the heart and the lungs. This objective is accomplished by cannulas that
drain blood from the right side of the heart and a cannula that allows perfusion
of the arterial circulation. However, this system alone does not effectively drain
all blood that enters the heart during surgery. Systemic-to-pulmonary collaterals,
systemic-to-systemic collaterals, and surgical site bleeding are routes of blood
return to the left side of the heart and into the surgical field that require specific
drainage mechanisms (cannulas) in the CPB pump to safely conduct CPB. Current CPB
pumps can also be used to deliver cardioplegia through an independent pump and tubing
mechanism. In addition to the pumping/suction mechanisms and the associated tubing
connections to the patient, the CPB pump has several features that can be broadly
categorized into three groups: (1) oxygenating-ventilating system that supplies
oxygen and removes carbon dioxide, (2) cooling-warming system, and (3) several in-line
monitors and safety devices ( Fig.
50-31
).
Venous drainage from the patient to the pump is usually effected
by gravity and is thus dependent on the height of the patient above the venous reservoir.
This passive mechanism of venous return to the pump is subject to the risk of airlock.
Suction mechanisms can also be applied to the venous lines to promote venous drainage.
However, this approach requires close monitoring since excessive suctioning can
paradoxically reduce venous outflow by causing collapse of the vena cava/right atrium
over the tips of the venous cannula. Patient circumstances and surgical preference
determine the type and location of venous cannula placement. Bicaval, single atrial,
single cavoatrial, and femoral venous cannulation can each be used to effect venous
drainage. Each approach has its advantages and disadvantages. For example, bicaval
cannulation of the inferior and superior vena cava with tourniquet placement is necessary
for mitral valve surgery, ensures adequate venous return, and minimizes return of
blood to the right side of the heart and the consequential myocardial rewarming,
but it is technically more difficult. Femoral venous cannulation can be used as
a temporizing measure if it is deemed necessary to decompress the heart during high-risk
repeat surgery. Arterial cannulas are usually placed in the ascending aorta, but
they can be placed in the femoral or axillary arteries, as dictated by circumstances.
Non-vena caval blood return to the right side of the heart (through
the coronary sinus before aortic cross-clamping, through the thebesian veins, and
via systemic-to-systemic collaterals) is usually effectively drained by the atrial
or cavoatrial cannula. Bicaval cannulation is often performed in circumstances in
which the right atrium is opened surgically (e.g., closure of atrial septal defects)
to minimize the risk of right ventricular distention. Distention of the left ventricle
is an ever-present risk during CPB with aortic cross-clamping. It occurs as a result
of bronchopulmonary collateral flow, systemic-to-systemic pericardial collateral
flow, and thebesian venous return to the ventricular cavity, especially when the
aorta is unclamped and there is the potential for coronary flow. Venous return to
the left ventricle results not only in left ventricular distention and potentially
subendocardial myocardial ischemia, but also in left ventricular warming (CPB with
hypothermia of ∼30°C still delivers blood to the heart that is warm relative
to myocardial temperatures during anoxic arrest induced by cardioplegic solutions
with temperatures between 17°C and 20°C). Thus, effective venting of the
left heart is an essential feature of CPB. The left side of the heart can be effectively
vented by cannulas placed in either the left ventricle, left atrium, right superior
pulmonary vein, or pulmonary artery. The patient's specific circumstances and practitioner
preferences
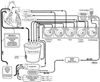 Figure 50-31
Illustration outlining the essential features of a typical
cardiopulmonary bypass circuit. Venous blood is drained into a venous reservoir
by gravity (although vacuum assist devices can be added) from the right atrium by
using either a single cannula or bicaval cannulation. Blood can also be salvaged
from the surgical field and cardiopulmonary cavities and returned to the venous reservoir
by cardiotomy suckers and appropriately placed vents, respectively, thus avoiding
increases in left ventricular pressure and left ventricular distention resulting
from collateral flow in the presence of aortic cross-clamping. Blood from the venous
reservoir is pumped by a nonocclusive roller-head pump through the oxygenator to
the patient. The content of the gas flow to the oxygenator determines the arterial
blood gas and acid-base content. Various monitoring and safety devices are shown
for these circuits. Cardioplegia can also be delivered by a roller-head pump and
is monitored as shown. Both cardioplegia and the patient's blood can be heated-cooled
by heat exchangers connected to a water-based heater-cooler device.
Figure 50-31
Illustration outlining the essential features of a typical
cardiopulmonary bypass circuit. Venous blood is drained into a venous reservoir
by gravity (although vacuum assist devices can be added) from the right atrium by
using either a single cannula or bicaval cannulation. Blood can also be salvaged
from the surgical field and cardiopulmonary cavities and returned to the venous reservoir
by cardiotomy suckers and appropriately placed vents, respectively, thus avoiding
increases in left ventricular pressure and left ventricular distention resulting
from collateral flow in the presence of aortic cross-clamping. Blood from the venous
reservoir is pumped by a nonocclusive roller-head pump through the oxygenator to
the patient. The content of the gas flow to the oxygenator determines the arterial
blood gas and acid-base content. Various monitoring and safety devices are shown
for these circuits. Cardioplegia can also be delivered by a roller-head pump and
is monitored as shown. Both cardioplegia and the patient's blood can be heated-cooled
by heat exchangers connected to a water-based heater-cooler device.
determine which site is chosen. The blood is actively evacuated by a roller-head
pump on the CPB machine and drained into the cardiotomy reservoir. The latter also
collects blood from a cardiotomy sucker, which is used to salvage blood from the
surgical field (see Fig. 50-31
).
Today, membrane oxygenators are used almost exclusively and usually
incorporate a venous reservoir, a heat exchanger, and gas flow conduits. Monitors
and safety devices are somewhat variable but include in-line filters and bubble traps,
in-line blood gas monitors, pressure and flow measurement mechanisms, oxygen and
gas flow measurement devices, temperature monitoring, and low-level sensor detectors
on the venous reservoir. CPB circuits can also incorporate an anesthetic vaporizer.
As previously mentioned, cardioplegia can be delivered by the CPB system ( Fig.
50-31
).
 Figure 50-31
Illustration outlining the essential features of a typical
cardiopulmonary bypass circuit. Venous blood is drained into a venous reservoir
by gravity (although vacuum assist devices can be added) from the right atrium by
using either a single cannula or bicaval cannulation. Blood can also be salvaged
from the surgical field and cardiopulmonary cavities and returned to the venous reservoir
by cardiotomy suckers and appropriately placed vents, respectively, thus avoiding
increases in left ventricular pressure and left ventricular distention resulting
from collateral flow in the presence of aortic cross-clamping. Blood from the venous
reservoir is pumped by a nonocclusive roller-head pump through the oxygenator to
the patient. The content of the gas flow to the oxygenator determines the arterial
blood gas and acid-base content. Various monitoring and safety devices are shown
for these circuits. Cardioplegia can also be delivered by a roller-head pump and
is monitored as shown. Both cardioplegia and the patient's blood can be heated-cooled
by heat exchangers connected to a water-based heater-cooler device.
Figure 50-31
Illustration outlining the essential features of a typical
cardiopulmonary bypass circuit. Venous blood is drained into a venous reservoir
by gravity (although vacuum assist devices can be added) from the right atrium by
using either a single cannula or bicaval cannulation. Blood can also be salvaged
from the surgical field and cardiopulmonary cavities and returned to the venous reservoir
by cardiotomy suckers and appropriately placed vents, respectively, thus avoiding
increases in left ventricular pressure and left ventricular distention resulting
from collateral flow in the presence of aortic cross-clamping. Blood from the venous
reservoir is pumped by a nonocclusive roller-head pump through the oxygenator to
the patient. The content of the gas flow to the oxygenator determines the arterial
blood gas and acid-base content. Various monitoring and safety devices are shown
for these circuits. Cardioplegia can also be delivered by a roller-head pump and
is monitored as shown. Both cardioplegia and the patient's blood can be heated-cooled
by heat exchangers connected to a water-based heater-cooler device.