Anesthetic Loss to Plastic and Soda Lime
Uptake of anesthetic by several depots also may hinder the development
of an adequate inspired anesthetic concentration. The rubber or plastic components
of the circuit may remove agent,[66]
[67]
and this was a significant problem with the obsolete anesthetic methoxyflurane, which
has a high solubility in rubber and plastic ( Table
5-3
). A minor problem exists for halothane and isoflurane, and no problem
results from the solution of nitrous oxide, desflurane, or sevoflurane.[68]
Normal (moist) carbon dioxide absorbents containing 13% to 15%
water can slightly increase the sevoflurane and, to a lesser extent, halothane requirements
by
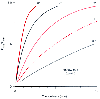 Figure 5-15
The rate at which the inspired anesthetic concentration
(FINS) rises toward the inflowing concentration (FINF)
is determined by the inflow rate and circuit volume. In the case illustrated, the
circuit volume is 7 L. (Adapted from Eger EI II: Effect of anesthetic circuit
on alveolar rate of rise. In Eger EI II [ed]: Anesthetic
Uptake and Action. Baltimore, Williams & Wilkins, 1974, pp 192–205.)
Figure 5-15
The rate at which the inspired anesthetic concentration
(FINS) rises toward the inflowing concentration (FINF)
is determined by the inflow rate and circuit volume. In the case illustrated, the
circuit volume is 7 L. (Adapted from Eger EI II: Effect of anesthetic circuit
on alveolar rate of rise. In Eger EI II [ed]: Anesthetic
Uptake and Action. Baltimore, Williams & Wilkins, 1974, pp 192–205.)
degrading each of these anesthetics.[69]
Degradation
results in the removal of hydrogen fluoride and the production of unsaturated compounds.
Nitrous oxide, desflurane, and isoflurane are not materially degraded. For sevoflurane
and halothane, degradation is of concern because the unsaturated compounds produced
are toxic (e.g., compound A from sevoflurane is nephrotoxic if given for prolonged
periods).[70]
[71]
In contrast to normal (moist) carbon dioxide absorbents, desiccated
absorbents materially degrade all potent inhaled anesthetics.[72]
Degradation of sevoflurane materially increases anesthetic requirement.[73]
Dehydration of Baralyme increases compound A production from sevoflurane, but dehydration
of soda lime does the opposite.[73]
Desiccated
absorbents degrade all anesthetics containing the CHF2
-O- moiety (i.e.,
desflurane, enflurane, and isoflurane) to carbon monoxide,[72]
but degradation by soda lime is minimal at hydrations approximately one tenth of
those found in normal soda lime (i.e., it only takes a little water to minimize degradation).
 Figure 5-15
The rate at which the inspired anesthetic concentration
(FINS) rises toward the inflowing concentration (FINF)
is determined by the inflow rate and circuit volume. In the case illustrated, the
circuit volume is 7 L. (Adapted from Eger EI II: Effect of anesthetic circuit
on alveolar rate of rise. In Eger EI II [ed]: Anesthetic
Uptake and Action. Baltimore, Williams & Wilkins, 1974, pp 192–205.)
Figure 5-15
The rate at which the inspired anesthetic concentration
(FINS) rises toward the inflowing concentration (FINF)
is determined by the inflow rate and circuit volume. In the case illustrated, the
circuit volume is 7 L. (Adapted from Eger EI II: Effect of anesthetic circuit
on alveolar rate of rise. In Eger EI II [ed]: Anesthetic
Uptake and Action. Baltimore, Williams & Wilkins, 1974, pp 192–205.)