Ventilation-Perfusion Ratio Abnormalities
Until this point, I have assumed equality of alveolar and arterial
anesthetic partial pressures (i.e., that the alveolar gases completely equilibrate
with the blood passing through the lungs). For normal patients, this assumption
is approximately correct, but diseases such as emphysema, pneumonia, and atelectasis,
as well as congenital cardiac defects, produce substantial deviations from equilibration.
The associated ventilation-perfusion ratio abnormality increases the alveolar (end-tidal)
anesthetic partial pressure and decreases the arterial anesthetic partial pressure
(i.e., a partial pressure difference appears between alveolar gas and arterial blood).
The relative change depends on the solubility of the anesthetic. With a poorly
soluble agent, the end-tidal concentration is slightly increased, but the arterial
partial pressure is significantly reduced. The opposite occurs with a highly soluble
anesthetic.[52]
The considerable decrease in the arterial anesthetic partial pressure
that occurs with poorly soluble agents may be explained as follows. Ventilation-perfusion
ratio abnormalities increase ventilation relative to perfusion of some alveoli, whereas
in other alveoli, the reverse occurs. With a poorly soluble anesthetic, an increase
in ventilation relative to perfusion does not appreciably increase the alveolar or
arterial anesthetic partial pressure issuing from those alveoli (see Fig.
5-5
for nitrous oxide effect). However, when ventilation decreases relative
to perfusion (e.g., with atelectasis), blood emerges from that segment with no additional
anesthetic. Such anesthetic-deficient blood then mixes with the blood from the ventilated
segments containing a normal complement of anesthetic. The mixture produces an arterial
anesthetic partial pressure considerably below normal.
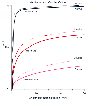 Figure 5-9
Proportional increases in alveolar ventilation (VA)
and cardiac output (Q) increase the rate at which the alveolar concentration of anesthetic/concentration
of inspired anesthetic (FA/FI
ratio) rises. The effect is relatively small if the increase in cardiac output is
distributed proportionately to all tissues (i.e., if cardiac output is doubled, all
tissue blood flows are doubled). The greatest effect occurs with the most soluble
anesthetic. (Adapted from Eger EI II, Bahlman SH, Munson ES: Effect of
age on the rate of increase of alveolar anesthetic concentration. Anesthesiology
35:365–372, 1971.)
Figure 5-9
Proportional increases in alveolar ventilation (VA)
and cardiac output (Q) increase the rate at which the alveolar concentration of anesthetic/concentration
of inspired anesthetic (FA/FI
ratio) rises. The effect is relatively small if the increase in cardiac output is
distributed proportionately to all tissues (i.e., if cardiac output is doubled, all
tissue blood flows are doubled). The greatest effect occurs with the most soluble
anesthetic. (Adapted from Eger EI II, Bahlman SH, Munson ES: Effect of
age on the rate of increase of alveolar anesthetic concentration. Anesthesiology
35:365–372, 1971.)
 Figure 5-10
The alveolar rate of rise of halothane concentration
is more rapid in children (dashed lines) than in
adults (solid lines). The difference probably results
from the greater ventilation and perfusion per kilogram of tissue in children and
the fact that a disproportionate amount of the increased perfusion is devoted to
the vessel-rich tissues. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Data from references [6]
[49]
[50]
and [51]
.)
Figure 5-10
The alveolar rate of rise of halothane concentration
is more rapid in children (dashed lines) than in
adults (solid lines). The difference probably results
from the greater ventilation and perfusion per kilogram of tissue in children and
the fact that a disproportionate amount of the increased perfusion is devoted to
the vessel-rich tissues. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Data from references [6]
[49]
[50]
and [51]
.)
With highly soluble agents, a different situation results from
the same ventilation-perfusion ratio abnormalities. In alveoli receiving more ventilation
relative to perfusion, the anesthetic partial pressure increases nearly in proportion
to the increase in ventilation (see Fig.
5-5
for effect with diethyl ether). Blood issuing from these alveoli has
an increased anesthetic content almost proportional to the increased ventilation.
Assuming that overall (total) ventilation remains normal, this increase in the anesthetic
contained by blood from the relatively hyperventilated alveoli compensates for the
lack of anesthetic uptake in unventilated alveoli.
These effects are illustrated in Figure
5-11
for endobronchial intubation. Direction of all ventilation to one
lung produces hyperventilation relative to perfusion. FA/FI
for this lung is slightly increased (above that obtained in the absence of endobronchial
intubation) with the poorly soluble cyclopropane and greatly increased with the highly
soluble ether. The increase with ether compensates for the absence of uptake from
the unventilated lung, a compensation that is not available with cyclopropane. The
result is that the cyclopropane arterial partial pressure is well below normal, whereas
the ether arterial partial pressure is scarcely changed.
These concepts have been confirmed experimentally by comparing
the rate of arterial anesthetic rise with
 Figure 5-11
When no ventilation-perfusion abnormalities exist, the
alveolar (PA) and arterial (Pa) anesthetic partial
pressures rise together (solid lines) toward the
inspired partial pressure (PI). When 50% of the
cardiac output is shunted through the lungs, the rate of rise of the end-tidal partial
pressure (dashed lines) is accelerated, and the rate
of rise of the arterial partial pressure (dotted or dashed lines)
is retarded. The greatest retardation occurs with the least soluble anesthetic,
cyclopropane. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Adapted from Eger EI II, Severinghaus JW: Effect of uneven pulmonary distribution
of blood and gas on induction with inhalation anesthetics. Anesthesiology 25:620–626,
1964.)
Figure 5-11
When no ventilation-perfusion abnormalities exist, the
alveolar (PA) and arterial (Pa) anesthetic partial
pressures rise together (solid lines) toward the
inspired partial pressure (PI). When 50% of the
cardiac output is shunted through the lungs, the rate of rise of the end-tidal partial
pressure (dashed lines) is accelerated, and the rate
of rise of the arterial partial pressure (dotted or dashed lines)
is retarded. The greatest retardation occurs with the least soluble anesthetic,
cyclopropane. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Adapted from Eger EI II, Severinghaus JW: Effect of uneven pulmonary distribution
of blood and gas on induction with inhalation anesthetics. Anesthesiology 25:620–626,
1964.)
and without endobronchial intubation in dogs.[53]
Endobronchial intubation significantly slowed the arterial rate of rise of cyclopropane
but did not influence the rise with methoxyflurane. An intermediate result was obtained
with halothane ( Fig. 5-12
).
These data suggest that in the presence of ventilation-perfusion ratio abnormalities,
the anesthetic effect of agents such as nitrous oxide, desflurane, and sevoflurane
may be delayed more than the anesthetic effect of isoflurane or halothane.
 Figure 5-9
Proportional increases in alveolar ventilation (VA)
and cardiac output (Q) increase the rate at which the alveolar concentration of anesthetic/concentration
of inspired anesthetic (FA/FI
ratio) rises. The effect is relatively small if the increase in cardiac output is
distributed proportionately to all tissues (i.e., if cardiac output is doubled, all
tissue blood flows are doubled). The greatest effect occurs with the most soluble
anesthetic. (Adapted from Eger EI II, Bahlman SH, Munson ES: Effect of
age on the rate of increase of alveolar anesthetic concentration. Anesthesiology
35:365–372, 1971.)
Figure 5-9
Proportional increases in alveolar ventilation (VA)
and cardiac output (Q) increase the rate at which the alveolar concentration of anesthetic/concentration
of inspired anesthetic (FA/FI
ratio) rises. The effect is relatively small if the increase in cardiac output is
distributed proportionately to all tissues (i.e., if cardiac output is doubled, all
tissue blood flows are doubled). The greatest effect occurs with the most soluble
anesthetic. (Adapted from Eger EI II, Bahlman SH, Munson ES: Effect of
age on the rate of increase of alveolar anesthetic concentration. Anesthesiology
35:365–372, 1971.) Figure 5-10
The alveolar rate of rise of halothane concentration
is more rapid in children (dashed lines) than in
adults (solid lines). The difference probably results
from the greater ventilation and perfusion per kilogram of tissue in children and
the fact that a disproportionate amount of the increased perfusion is devoted to
the vessel-rich tissues. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Data from references [6]
[49]
[50]
and [51]
.)
Figure 5-10
The alveolar rate of rise of halothane concentration
is more rapid in children (dashed lines) than in
adults (solid lines). The difference probably results
from the greater ventilation and perfusion per kilogram of tissue in children and
the fact that a disproportionate amount of the increased perfusion is devoted to
the vessel-rich tissues. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Data from references [6]
[49]
[50]
and [51]
.) Figure 5-11
When no ventilation-perfusion abnormalities exist, the
alveolar (PA) and arterial (Pa) anesthetic partial
pressures rise together (solid lines) toward the
inspired partial pressure (PI). When 50% of the
cardiac output is shunted through the lungs, the rate of rise of the end-tidal partial
pressure (dashed lines) is accelerated, and the rate
of rise of the arterial partial pressure (dotted or dashed lines)
is retarded. The greatest retardation occurs with the least soluble anesthetic,
cyclopropane. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Adapted from Eger EI II, Severinghaus JW: Effect of uneven pulmonary distribution
of blood and gas on induction with inhalation anesthetics. Anesthesiology 25:620–626,
1964.)
Figure 5-11
When no ventilation-perfusion abnormalities exist, the
alveolar (PA) and arterial (Pa) anesthetic partial
pressures rise together (solid lines) toward the
inspired partial pressure (PI). When 50% of the
cardiac output is shunted through the lungs, the rate of rise of the end-tidal partial
pressure (dashed lines) is accelerated, and the rate
of rise of the arterial partial pressure (dotted or dashed lines)
is retarded. The greatest retardation occurs with the least soluble anesthetic,
cyclopropane. FA/FI
is the alveolar concentration of anesthetic/concentration of inspired anesthetic.
(Adapted from Eger EI II, Severinghaus JW: Effect of uneven pulmonary distribution
of blood and gas on induction with inhalation anesthetics. Anesthesiology 25:620–626,
1964.)