|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Whether the pharmacology of local anesthetics is similar in children and in adults, there are certain differences that must be considered before a regional block is performed, especially in neonates and infants[8] (see Chapter 14 ). The effects of local anesthetics depend on their spread from the site of injection, which is often considerable in infants, in the epidural space and along the nerve sheath; their fixation at the local binding sites such as surface proteins and lipids, especially myelin; and the permeability of nerve fibers. In young patients, the endoneurium is loose and easily traversed in both directions. As the child grows up, the endoneurium becomes enriched in connective fibers and becomes less permeable. The latency and duration of nerve blockade increase with age.
In their nonionized form, local anesthetics cross almost freely the endothelium of the capillaries surrounding the site of injection. Because the cardiac output and the local blood flow of infants are two to three times greater than in adults, the systemic absorption of local anesthetics is increased accordingly, and vasoactive agents such as epinephrine are very effective in slowing the systemic uptake.
For topical anesthesia, early studies demonstrated a very rapid rise in plasma concentrations in younger children, which was not confirmed by later studies, and the recommendations for topical anesthesia use have changed.[9] [10]
Compartment blocks such as intercostal nerve, intrapleural, paravertebral, ilioinguinal nerve, and fascia iliaca compartment blocks offer a large surface for the absorption of local anesthetics, which favors early and high peak plasma concentrations (i.e., short Tmax and high Cmax ). This is well demonstrated in adults with intercostal nerve blocks (Tmax near 10 versus 30 minutes after epidural administration)[11] and in infants with interpleural blocks.[12] Conversely, more distal techniques such as ilioinguinal nerve and fascia iliaca compartment blocks do not lead to high Cmax , even after continuous infusion, which could preclude their use in infants and children.[13] [14]
Conduction blocks (especially epidural anesthesia) have various absorption patterns. Aminoamides bind to lipidic molecules, especially the epidural fat, and their absorption into the bloodstream follows a biphasic process. Lidocaine is rapidly absorbed when administered extravascularly, and its blood concentration rapidly increases after repeat injections. Conversely, bupivacaine and ropivacaine are more strongly retained at the injection site, and their blood concentrations increase more slowly when multiple injections are performed. [15] [16] In adults, this slow absorption process combined with the buffering properties of serum proteins and red cells results in a progressive and smooth increase in blood concentration of long-lasting local anesthetics, which decreases toxicity. This slow process also influences pharmacokinetic parameters, leading to a decrease in plasma concentration slower than expected from data obtained after a single injection. The half-life and distribution volume increase with repeat extravascular administration. This is called the flip-flop effect. In children, the same applies to bupivacaine but not to ropivacaine. After an epidural injection (i.e., caudal or lumbar epidural), the Tmax of bupivacaine is nearly the same as in adults (about 30 minutes). Conversely, the Tmax of ropivacaine is much longer in infants (115 minutes) than in 3- to 5-year-old children (62 minutes). Adult values (30 minutes) are attained only between 5 and 8 years of age.[17] [18] Surprisingly, concomitant Cmax values are also higher in infants, ranging from 0.52 mg/L (1 to 2 years) to 0.47 mg/L (3 to 4 years) to 0.42 mg/L (5 to 8 years),[17] although a decrease of Cmax values was expected in association with delayed Tmax values (in case of constant clearance). The most probable explanation for this paradoxical effect is related to the immaturity of the liver. The lower clearance observed in infants is probably a major contributing factor to this late peak concentration after caudal injection. The intrinsic vasoconstrictive properties of (S)-isomers (i.e., levobupivacaine and ropivacaine) may also play a significant role, similar to that caused by epinephrine.[19] [20]
Within the bloodstream, local anesthetics distribute in the plasma and the red blood cells, with a blood-to-plasma concentration ratio ranging from 0.60 to 0.85,[21] [22] meaning that erythrocytes account for 20% to 30% of the total amount in blood, depending on the agent and the hematocrit. In normal conditions, red cells do not act as a buffering compartment, but their buffering capacities can become clinically relevant in infants with physiologic anemia, when binding to serum proteins is saturated (i.e., at toxic blood concentrations)[23] and in neonates, whose hematocrit may exceed 70% and whose erythrocytes are enlarged (i.e., physiologic macrocytosis).
In plasma, local anesthetics bind to α1 -acid glycoprotein (AAG) and to human serum albumin (HSA). For AAG (i.e., orosomucoid), the plasma concentration is low at birth (0.2 to 0.3 g/L) and progressively increases to the usual level of 0.7 to 1.0 g/L after the age of 1 year.[24] Because of this low concentration, there is a significant increase in the unbound, free form of all aminoamides with subsequent danger of systemic toxicity. AAG is a stress protein, the plasma concentration of which increases during inflammatory disorders, including the postoperative period,[22] [25] resulting in a rapid decrease in the free fraction of any local anesthetic during the first 3 to 6 hours after surgery. However, because long-acting anesthetics (e.g., bupivacaine, ropivacaine) have a rather low hepatic extraction ratio (discussed later), this decrease in the free fraction is concomitant with a parallel decrease in hepatic clearance. There is no significant change in the concentration of the unbound form of these local anesthetics.
Local anesthetics can bind to HSA and can compete with molecules previously bound to HSA. The affinity of HSA for local anesthetics is about 5000 to 10,000 times lower than that of AAG; it is only when the binding capacity of AAG has become saturated that HSA may play a role similar to that of red blood cells.
| Drugs | Protein Binding (%) | Vdss (L/kg) | Clearance (mL/kg/min) | Elimination Half-Life (hr) | Elimination in Unchanged Form (%) |
|---|---|---|---|---|---|
| Lidocaine |
|
|
|
|
|
| Neonate | 25 | 1.4–4.9 | 5–19 | 2.9–3.3 | 0.16 |
| Adult | 55–65 | 0.2–1.0 | 11–15 | 1.0–2.2 | 0.02 |
| Mepivacaine |
|
|
|
|
|
| Neonate | 36 | 1.2–2.8 | 1.6–3 | 5.3–11.3 | 0.36 |
| Adult | 75–80 | 0.6–1.5 | 10–13 | 1.7–6.9 | 0.04 |
| Bupivacaine |
|
|
|
|
|
| Neonate | 50–70 | 3.9 | 7 | 6.0–22.0 | — |
| Adult | 85–95 | 0.8–1.6 | 7–9 | IV: 1.2–2.9 | 0.03 |
|
|
|
|
|
Epidural: 5–10 |
|
| Ropivacaine |
|
|
|
|
|
| Infants | 94 | 2.4 | 6.5 | — | — |
| Adult | 94 | 1.1 ± 0.25 | 10–13 | 2.41 ± 0.52 | 1 |
| IV, intravenous; Vdss , distribution volume at the steady state. | |||||
Protein binding can be affected by several conditions: the isomeric form of the local anesthetic, with (S)-enantiomers being more tightly bound[26] [27] ; the existence of acidosis, which decreases protein binding in a clinically relevant manner[28] ; and competition with other agents or biologic products bound to plasma proteins, which is rare[29] and involves mainly β-blockers, calcium channel blockers, and other amide local anesthetics.[30]
After systemic absorption and protein binding, local anesthetics undergo distribution to different body fluid compartments and tissues. Body fluid compartments vary with the patient's age. Water accounts for 80% of body weight in premature infants, 75% in term neonates, 65% in infants, and 60% in older children and adults. Concomitantly, the relative importance of body fluid compartments changes considerably. Intracellular fluids increase from 20% of body weight in premature infants to 30% in adolescents, and extracellular fluids are reduced by 50% from birth to adulthood. The pharmacokinetics of local anesthetics is strongly affected by these changes ( Table 45-2 ). The distribution volume of all agents is markedly increased in the very young. The peak plasma concentration after injection of a single given dose is less in infants than in adults, reducing toxicity and counteracting the increased systemic absorption associated with greater local blood flow. At the same time, during the first 2 years of life, the clearance of all aminoamides is low,[24] [31] and their half-lives are considerably increased. This leads to drug accumulation in the case of repeated injections.[32] [33] [34] [35] By the second year of life, the clearance of aminoamides increases progressively, becoming higher than that of adults and allowing young children to tolerate doses of local anesthetics that would be toxic in adults. However, this peculiarity should not encourage the administration of excessive doses of local anesthetics.
With the increasing development of continuous infusion techniques, it is important to consider pharmacokinetic parameters after injection of a single dose and after repeat or continuous infusions. This is critical for long-lasting local anesthetics. Ropivacaine does not follow the same pattern as bupivacaine in infants and young children. The distribution volume of bupivacaine after a single injection is higher than that of ropivacaine throughout childhood, but it consistently decreases during continuous infusion and becomes less than that of ropivacaine, which concomitantly tends to increase ( Fig. 45-1 ).[36]
After systemic distribution, the local anesthetic is progressively eliminated by plasma or hepatic metabolism, and a small amount is excreted in the unchanged form in the urine and in gastric secretions, especially in neonates.
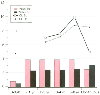 Figure 45-1
Mean variations of Vdss
(L/kg) and clearance
(mL/kg/min) of bupivacaine (Bu) and ropivacaine (Ro) according to the patient's age
(after a single injection) and during continuous epidural infusion in young children.
Vdss
, distribution volume at the steady state.
Figure 45-1
Mean variations of Vdss
(L/kg) and clearance
(mL/kg/min) of bupivacaine (Bu) and ropivacaine (Ro) according to the patient's age
(after a single injection) and during continuous epidural infusion in young children.
Vdss
, distribution volume at the steady state.
The hepatic extraction ratio of lidocaine ranges from 0.65 to 0.75. Its systemic elimination is flow limited rather than rate limited, and any decrease in cardiac output significantly reduces its hepatic clearance.[48] Continuous infusion of lidocaine has the same effect, leading to a dramatic decrease in intrinsic clearance,[49] [50] reinforced by the fact that its metabolism is impaired by its own metabolites.[49] Lidocaine is not recommended for continuous infusions, especially because tachyphylaxis occurs more often with lidocaine than with any other local anesthetic. Bupivacaine and ropivacaine have a low hepatic extraction ratio (0.30 to 0.35), and their elimination is rate limited. A change in protein binding is the major factor that affects their total clearance (considering that their intrinsic clearance is constant). Protein binding increases during the postoperative period because of the increase in AAG concentration in serum, and a parallel decrease in clearance is observed.[22] However, this leads to a resetting of the total serum concentration, and the unbound concentration remains constant. The total-body clearance of bupivacaine and ropivacaine is similar for bupivacaine and ropivacaine after a single injection. It is low at birth and progressively increases during the first years of life.[46] [47] After continuous infusion, the two local anesthetics behave differently. The clearance of ropivacaine remains unchanged, whereas that of bupivacaine decreases by more than 40% (see Fig. 45-1 ).[22]
Toxic plasma levels of local anesthetics are largely unknown and based on assumptions derived from anecdotal case reports. The administration of common doses of local anesthetics (i.e., 6 to 8 mg/kg of lidocaine or mepivacaine and 1.5 to 2 mg/kg of bupivacaine) leads to peak plasma concentration (bound and unbound forms) ranging from 3 to 5 µg/L for lidocaine or mepivacaine and 0.5 to 1 µg/L for bupivacaine.[24] Clinical signs of toxicity have been reported with plasma concentration ranging from 7 to 10 µg/L for lidocaine or mepivacaine and from 1.5 to 2 µg/L (intraoperatively) and from 2 to 2.5 µg/L (postoperatively) with bupivacaine. However, plasma concentrations of bupivacaine exceeding 4 µg/L have been commonly detected without any clinical sign of toxicity. The toxic form of all local anesthetics is the free, unbound form, which is difficult to measure and correlates poorly with measured concentrations. Three studies done in adult volunteers[51] [52] [53] corroborate animal studies showing that ropivacaine and levobupivacaine are less toxic than racemic bupivacaine. From these studies, it is considered that the threshold of toxicity is about 0.3 µg/L of unbound bupivacaine and 0.6 µg/L of unbound ropivacaine or levobupivacaine in adults. Infants and young children seem less prone than adults to develop central nervous system toxicity, but they are more prone to develop cardiac toxicity.[54] Neonates and young infants are considered more sensitive to toxicity because of a lower serum binding.
Lidocaine and mepivacaine are short-acting agents that should be used for single-injection techniques only. Bupivacaine and ropivacaine have advantages and limitations. Ropivacaine has less intrinsic toxicity than bupivacaine, but after a single injection, its Tmax is delayed, and somewhat illogically, its Cmax is concomitantly increased (requiring longer monitoring of the patient after injection). After continuous infusion, bupivacaine undergoes a consistent decrease in distribution volume and clearance, whereas the distribution volume of ropivacaine increases slightly, and its clearance remains stable, which makes its use preferable in this condition.
Whether the pharmacokinetics of narcotics has no special features in infants and children,[55] [56] there are some concerns related to pharmacodynamics, especially in neonates.[57] Opioids bind specifically to μ, κ, and δ receptors located in the brain, spinal cord, and other tissues. The resulting conformational changes in the receptors initiate signal transduction machinery involving inhibitory G proteins and leading to hyperpolarization of the neuronal membrane, shortening the action potential's duration and decreasing neurotransmitter release. The final result is sedation, analgesia, and respiratory depression. When submitted to repeat opioid stimulation, opioid receptors undergo desensitization and uncoupling from G proteins. [58]
During critical periods of development, experimental administration of a single dose or few doses of opioid analgesics may induce opioid tolerance in neonates,[59] [60] [61] which develops more rapidly in preterm than in term neonates.[62] Differences in metabolism of morphine may contribute to this effect. In preterm neonates, morphine primarily yields morphine-3-glucuronide, which has antianalgesic properties, instead of morphine-6-glucuronide, which accumulates because its half-life is longer than that of morphine. Gender plays a significant role, and male preterm rats develop more intense tolerance than female preterm rats.[63] Similar differences in the response to pain of male and female preterm human neonates have been reported. [64] The development of tolerance is greater after administration of synthetic opioids,[65] but the underlying cellular mechanism is unknown. NMDA receptors are involved in the development of opioid tolerance because NMDA antagonists can prevent its development.[66]
After intrathecal administration,[67] morphine reaches high concentrations in the cerebrospinal fluid (CSF), whereas the plasma concentration remains very low, making it difficult to estimate the half-life. After lumbar epidural injection, systemic uptake of morphine is comparable to that after intramuscular injection.[68] [69] Peak plasma concentration is reached within 10 minutes but does not account for the pharmacodynamic effects, which depend on the drug crossing the dura mater. Between 15 and 20 hours after epidural injection, the CSF concentration of morphine is 50 to 250 times higher than that of plasma. The elimination half-life from CSF is similar to that of plasma, following the same monoexponential elimination curve, but because original concentrations are very high, it takes 12 to 24 hours before the spinal concentration falls below the minimal effective concentration (approximately 10 ng/mL). Because morphine is hydrosoluble, its distribution volume, when administered epidurally, equals that of the CSF volume. Addition of epinephrine to the narcotic solution decreases systemic uptake and increases the CSF concentration of morphine,[70] which results in a slight but significant increase in duration of pharmacodynamic effects.
Morphine injection along the neuraxis is indicated for long-lasting
pain relief. It improves the outcome of many major operations on healthy but also
high-risk pediatric patients.[71]
However, as in
adults, adverse effects occur in about 50% of patients. These consequences include
pruritus, nausea, vomiting, urinary retention, and respiratory depression, which
can be delayed several hours after the injection, making postoperative monitoring
of respiratory parameters mandatory for the first 24 hours. The use of apnea monitors
or pulse oximeters, or both, can be helpful but cannot replace hourly clinical evaluation;
neonates should preferably be monitored in specialized neonatal units. Respiratory
depression is almost always preceded by generalized pruritus and sedation, which
must be carefully identified before the respiratory rate progressively
| Additive | Usual Dosages | Adverse Effects |
|---|---|---|
| Morphine |
|
|
| Intrathecal | 10 µg/kg | Pruritus, nausea and vomiting, urinary retention, delayed respiratory depression, sedation, constipation |
| Epidural | 30 µg/kg |
|
| Short-acting narcotics (epidural blocks only) |
|
|
| Fentanyl | 1–2 µg/kg | Pruritus, nausea and vomiting, urinary retention, early respiratory depression (apnea), sedation |
| Sufentanil | 0.5 µg/kg |
|
| Clonidine (neuraxial or peripheral blocks) | 1–1.5 µg/kg | Sedation (slight) |
|
|
|
Hypotension at doses exceeding 2 µg/kg |
|
|
|
Possible respiratory depression in premature infants and neonates |
| Ketamine (epidural blocks) | 0.5 mg/kg | Sedation (but no behavioral disturbance) |
Short-acting opioids such as fentanyl (1 to 2 µg/kg) and sufentanil (0.5 µg/kg) can be administered epidurally instead of morphine. They improve intraoperative analgesia, especially when diluted solutions of local anesthetics are used, but have little effect on the prolongation of postoperative pain relief unless a continuous infusion or reinjections are made. Part of their action results from systemic effects after vascular absorption, which can cause early respiratory depression (conversely, delayed respiratory depression is unlikely).
Epinephrine (5 mg/L or 1:200,000) is the additive the most commonly combined with local anesthetics with no or little intrinsic vasoconstrictive properties such as lidocaine, mepivacaine, bupivacaine, and levobupivacaine, but not ropivacaine (see Table 45-3 ). This addition results in decreasing the plasma peak concentration[74] [75] and increasing the duration of postoperative analgesia in children younger than 4 years.[76] It has been suggested that such an addition of epinephrine might decrease the spinal cord blood flow in infants, possibly leading to neurologic deficits.[77] This assertion proved to be unfounded.[78] Nevertheless, many physicians prefer using epinephrine at lower concentrations (2.5 mg/L or 1:400,000) in small infants, which has been effective in delaying absorption of 0.25% bupivacaine administered by the caudal route.[79] Epinephrine allows detection of an inadvertent intravascular injection by producing early (within 20 seconds) ST segment elevation, T-wave change,[80] and hypertension.[81]
Clonidine is an α2 -adrenergic agonist (like epinephrine) that is increasingly used as an adjuvant to local anesthetics
Ketamine, especially (S)-ketamine, is undergoing renewed interest as an additive to local anesthetics because of its effects on NMDA receptors and its interaction with sodium channels in a local anesthetic-like fashion, including sharing a binding site with commonly used clinical local anesthetics.[87] The local administration of a solution containing 0.25 to 0.5 mg/kg provides a long-lasting analgesic effect[88] [89] with no significant, especially behavioral, adverse effects.
Many other additives have been administered along with local anesthetics, often prompting ethical questions. Among those of potential interest are corticosteroids, buprenorphine, neostigmine, tramadol, midazolam, and biodegradable bupivacaine or polyester microspheres. However, these adjuvants have not been approved for use in pediatric patients, and some may be more detrimental than beneficial.
|
|
|
|
|
|
|
|
|
|
|
|
|