Stewart-Fencl Approach
A more accurate reflection of true acid-base status can be derived
using the Stewart-Fencl approach.[1]
[2]
This is based on the concept of electrical neutrality, a small advance from using
the AG. There exists in plasma a SID of [(Na+
+ Mg2+
+ Ca2+
= K+
) − (Cl−
+ A−
)] of 40 to 44m Eq/L, balanced by the negative
charge on bicarbonate and ATOT
(the buffer base). There is a small difference
between SIDa (apparent SID) and buffer base (effective SID [SIDe]). This represents
a strong ion gap (SIG), which quantifies the amount of unmeasured anions present
( Fig. 41-5
).
SIDa = ([Na+
] + [K+
]
+ [Mg2+
] + [Ca2+
]) − [Cl−
]
SIDe = [HCO3
−
] + (charge
on albumin) + (charge on inorganic phosphate [Pi]) (in mmol/L)
Weak acids' degree of ionization is pH dependent, and calculations must include this:
[alb] = [alb] (in g/L) × (0.123 × pH −
0.631)
[Pi] (in mg/dL) = [Pi] / 10 × pH − 0.47.
SIG = SIDa − SIDe
The weakness of this system is that the SIG does not necessarily represent unmeasured
strong anions but merely
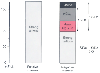 Figure 41-5
The strong ion gap apparent (SIDa) is the sum of the
total concentration of weak ions (ATOT
), such as albumin (Alb) and inorganic
phosphate (Pi), plus the concentration of bicarbonate ions [HCO3
-
].
SID effective (SIDe) is the real SID. The difference between the two is made up
of unmeasured anions (UMAs).
Figure 41-5
The strong ion gap apparent (SIDa) is the sum of the
total concentration of weak ions (ATOT
), such as albumin (Alb) and inorganic
phosphate (Pi), plus the concentration of bicarbonate ions [HCO3
-
].
SID effective (SIDe) is the real SID. The difference between the two is made up
of unmeasured anions (UMAs).
all anions that are unmeasured. SID changes quantitatively in absolute and relative
terms when there are changes in plasma water concentration. Fencl addressed this
problem by correcting the chloride concentration for free water, [Cl-
]
corrected with the following equation:
[Cl−
]corrected = [Cl−
]observed
× ([Na+
]normal/[Na+
]observed)
This corrected chloride concentration may be then inserted into the previous SIDa
equation. Likewise, the derived value for UMAs should also be corrected for free
water using UMAs instead of Cl-
in the previous equation.[18]
In a series of nine normal subjects, Fencl estimated the normal SIG as 8
± 2 mEq/L.[18]
Although accurate, the SIG is cumbersome and expensive, requiring
measurement of multiple ions and albumin. An alternative approach, used by Gilfix
and colleagues[31]
and subsequently by Balasubramanyan
and coworkers,[32]
is to calculate the BD or base
excess gap (BEG). This allows recalculation of BDE using strong ions, free water,
and albumin. The resulting BEG (i.e., BE caused by UMAs) should mirror the SIG and
AG, and these terms may be manipulated to provide the following equations and definitions:
BEG = BDE − CBE
BDE = Standard BDE
CBE = Calculated BDE
BEfw = Changes in free water = 0.3 × (Na
− 140)
BECl = Changes in chloride = 102 − (Cl −
140/Na)
BEalb = Changes in albumin = 3.4 × (4.5 −
albumin)
CBE = BEfw + BECl + BEalb
It is probable that no single number will ever allow us to make sense of complex
acid-base disturbances. Fencl[18]
has suggested
that, rather than focusing on AG or BDE, physicians should address each blood gas
in terms of all alkalinizing and acidifying effects: respiratory acidosis or alkalosis,
the presence or absence of abnormal SID (due to water excess or deficit, measured
electrolytes or unmeasured electrolytes), and abnormal ATOT
. This does
not necessarily mean that when examining a blood gas result and serum chemistry profile,
the physician is required to perform a series of calculations. Many acid-base abnormalities
can be inferred by "eyeballing" the laboratory values[18]
:
- Hyperchloremic acidemia is usually present if the corrected serum chloride
level is more than 112 mEq/L.
- Hypochloremic alkalemia is usually present when the corrected serum chloride
level is less than 100 mEq/L.
- Dilutional acidemia is usually present when the serum sodium level is less
than 136 mEq/L.
- Contraction alkalemia is usually present when the serum sodium level is
more than 148 mEq/L.
- Hyperphosphatemic acidemia is usually present when the serum phosphate
level is more than 2.0 mmol/L.
- Hypoalbuminemic alkalosis is usually present when the serum albumin level
is less than 3.5 g/dL.
Although this approach may appear inelegant, it has the advantage
of being comprehensive. Consider the following data for a patient described by Fencl
[18]
(in mEq/L unless
otherwise stated): Na = 117, K = 3.9, Ca = 3.0, Mg = 1.4, Cl = 92, Pi = 0.6 mmol/L,
albumin = 6.0 g/L, pH = 7.33, PCO2
= 30
mm Hg, HCO3
= 15, AG = 13, AGcorrected
= 23, BE = -10, SID
= 18, Clcorrected
= 112, and UMAcorrected
= 18. Using traditional
methodology, this would be described as a non-AG metabolic acidosis, and the physician
would look for causes of bicarbonate wasting, such as renal tubular acidosis or gastrointestinal
losses. The degree of respiratory alkalosis is appropriate for the degree of acidosis
(ΔBD = ΔPCO2
). However, the
Fencl-Stewart method reveals a much more complex situation. SID is reduced to 18
mEq/L, caused by free water excess, UMAs, and surprisingly, hyperchloremia (see the
corrected chloride value). However, the degree of acidosis does not mirror this
metabolic disturbance because of the alkalizing force at play: hypoalbuminemia.
The corrected AG mirrors the change in SID, but this is grossly underestimated by
the BD. This patient has a dilutional acidosis, a hyperchloremic acidosis, and a
lactic acidosis!
For most patients presenting to the emergency room or operating
suite who have been previously healthy, the use of tools such as the BD or AG to
assess metabolic disturbances remains reasonable. However, for critically ill patients,
the most effective method of interpreting acid-base conundrums involves unraveling
synchronous acidifying and alkalinizing processes and using calculations or rules
of thumb to distinguish between the various forces at play. Unfortunately, a clinician's
ability to interpret such information depends on the amount of available data. A
simple blood gas determination alone may camouflage a significant acid-base disturbance.
 Figure 41-5
The strong ion gap apparent (SIDa) is the sum of the
total concentration of weak ions (ATOT
), such as albumin (Alb) and inorganic
phosphate (Pi), plus the concentration of bicarbonate ions [HCO3
-
].
SID effective (SIDe) is the real SID. The difference between the two is made up
of unmeasured anions (UMAs).
Figure 41-5
The strong ion gap apparent (SIDa) is the sum of the
total concentration of weak ions (ATOT
), such as albumin (Alb) and inorganic
phosphate (Pi), plus the concentration of bicarbonate ions [HCO3
-
].
SID effective (SIDe) is the real SID. The difference between the two is made up
of unmeasured anions (UMAs).