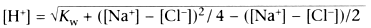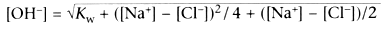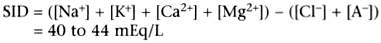Strong Ions
The strong ions, the first group of ions, are relatively simple
to incorporate because they dissociate completely. The most abundant strong ions
in the extracellular space are Na+
and Cl-
. Other important
strong ions include K+
, SO4
2-
, Mg2+
,
and Ca2+
. In a solution containing strong ions created, for example,
using specified concentrations of NaOH and HCl, the hydrogen ion concentration can
be calculated by solving for electric neutrality:
([Na+
] − [Cl−
]) +
([H+
] − [OH−
]) = 0
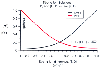 Figure 41-1
Effect of changes in the strong ion gap (SID) on hydrogen
and hydroxyl ion concentration. (Adapted from Stewart PA: Modern quantitative
acid-based chemistry. Can J Physiol Pharmacol 61:1444–1461, 1983.)
Figure 41-1
Effect of changes in the strong ion gap (SID) on hydrogen
and hydroxyl ion concentration. (Adapted from Stewart PA: Modern quantitative
acid-based chemistry. Can J Physiol Pharmacol 61:1444–1461, 1983.)
This creates two simultaneous equations:
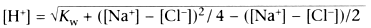
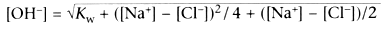
These equations tell us that hydrogen and hydroxyl concentrations are determined
by the Kw and the difference in charge between sodium and chloride. Because the
former is constant, in this system, ([Na+
− [Cl−
])
must determine [H+
] and [OH-
]. Because the concentration of
sodium and chloride are known, the net positive charge minus net negative charge
can be quantified. It is the strong ion difference (SID). In any solution, the
sum total of the charges imparted by strong cations minus the charges from strong
anions represets the SID. The SID independently influences hydrogen ion concentration
( Fig. 41-1
). In human ECF,
the SID is positive.
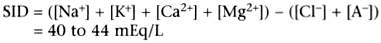
The pioneering work of Peter Stewart revealed several facts regarding [H+
]
that had not previously been understood. SID is always positive in human ECF, and
hydroxyl ions almost always exceed hydrogen ions quantitatively in solution. The
relationship between the SID and [H+
] is nonlinear in these conditions.
Any change in the SID changes the [H+
] and [OH-
] concentrations.
Because of the water dissociation constant, this relationship is inverse: as [H+
]
increases, [OH-
] decreases. SID is an independent variable, and [H+
]
and [OH-
] are dependent, meaning that the addition of hydrogen ions alone
(without strong corresponding anions) cannot influence the pH of the solution.
 Figure 41-1
Effect of changes in the strong ion gap (SID) on hydrogen
and hydroxyl ion concentration. (Adapted from Stewart PA: Modern quantitative
acid-based chemistry. Can J Physiol Pharmacol 61:1444–1461, 1983.)
Figure 41-1
Effect of changes in the strong ion gap (SID) on hydrogen
and hydroxyl ion concentration. (Adapted from Stewart PA: Modern quantitative
acid-based chemistry. Can J Physiol Pharmacol 61:1444–1461, 1983.)