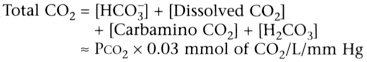ACIDS AND BASES
The concept of acids and bases is relatively new. In the early
part of the 20th century, it was known that the carbon dioxide (CO2
) content
of the blood decreased in cases of critical illness. As early as 1831, O'Shaughnessy
identified loss of "carbonate of soda" from the blood as a fundamental disturbance
in patients dying of cholera.[2]
We now know that
the loss of bicarbonate was related to hyperventilation and buffering of free hydrogen
ions in dysoxic or dysmetabolic states. In 1909, L.J. Henderson coined the term
acid-base balance.[6]
He was able to define this process in terms of carbonic acid equilibrium, work that
was later refined by Hasselbalch (1916).[7]
Essentially,
their method described acid-base balance in terms of the hydration equation for carbon
dioxide.[4]
The latter was the only clinical chemistry
test available at that time.
CO2
+ H2
O → H2
CO3
→ H+
+ HCO3
−
pH = pKa
+
log [HCO3
−
] / [H2
CO3
]
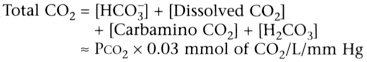
Substituting into the first previous equation provides the Henderson-Hasselbalch
equation:
pH = 6.1 + log [HCO3
−
]
/ PCO2
× 0.03
The revolutionary theory of Svante Arrhenius (1859–1927) in 1903 established
the foundations of acid-base chemistry. In an aqueous solution, an Arrhenius acid
is any substance that delivers a hydrogen ion into the solution.[2]
A base is any substance that delivers a hydroxyl ion into the solution. Water is
a highly ionizing solution because of its high dielectric constant, and substances
with polar bonds dissociate into their component parts (i.e., dissolve) in it. Hydrogen
chloride (HCl) is an acid, and potassium hydroxide (KOH) is a base.
The degree of dissociation of substances in water determines whether
they are strong acids or strong bases. Lactic acid, which has an ion dissociation
constant (pKa
) of 3.4, is completely dissociated
at physiologic pH and is a strong acid. Carbonic acid, which has a pKa
of 6.4, is incompletely dissociated and is a weak acid. Similarly, ions such as
sodium, potassium, and chloride, which do not easily bind other molecules, are considered
strong ions; they exist free in solution. In any solution, the ion dissociation
constant for water (Kw) dictates that the relative ratio of [H+
] to [OH-
]
must always be constant, and electrical neutrality must always hold. Consequently,
strong cations (Na+
, K+
, Ca2+
, Mg2+
)
act as Arrhenius bases because they drive hydroxyl out of and hydrogen into solution
to maintain electrical neutrality, and strong anions (Cl-
, LA-
[lactate], ketones, sulfate, formate) act as Arrhenius acids.
One problem with the Arrhenius theory is that it is not comprehensive
enough. Aqueous solutions such as ammonia (NH3
), sodium carbonate (Na2
CO3
),
and sodium bicarbonate (NaHCO3
) are bases, but none is a hydroxide. In
1923, Brønsted and Lowry proposed an expanded theory of acids and bases.
They defined acids as proton donors and bases as proton acceptors. All Arrhenius
acids and bases were therefore also Brønsted-Lowry acids and bases, and the
peculiar behavior of carbon dioxide and ammonia could be accounted for.
NH3
+ H2
O ⇌ NH4
+
+ OH−
In this situation, water is the proton donor, the Brønsted-Lowry acid, and
ammonia the proteon acceptor, the Brønsted-Lowry base. Conversely, consider
the following reaction:
HCl + H2
O → H3
O+
+ Cl−
In the previous reaction, hydrogen chloride acts as a Brønsted-Lowry acid
and water as a Brønsted-Lowry base.
CO2
+ H2
O ⇌ H2
CO3
⇌ H+
+ HCO3
−
In this reaction, carbon dioxide is hydrated to carbonic acid, a Brønsted-Lowry
acid, which subsequently dissociates to hydrogen (H+
) and bicarbonate
(HCO3
-
) ions.